Abstract
Molecular medicine is transforming everyday clinical practice from an empirical art to a rational ortho-molecular science. The prevailing concept in this emerging framework of molecular medicine is a personalized approach to disease prevention, diagnosis, prognosis, and treatment. In this mini-review, we discuss the educational and social-ethical issues raised by the advances of biomedical research as related to medical practice; outline the implications of molecular medicine for patients, physicians, and researchers; and underline the responsibilities of academia and the pharmaceutical industry to translate the scientific knowledge to a meaningful improvement of the quality of life across all members of society.
Similar content being viewed by others
Molecular medicine is transforming everyday clinical practice in an unprecedented manner (1). Oncologists treat RAS-mutated non-small cell lung cancer, hematologists encounter T315I-mutation chronic myelogenous leukemia, pathologists recognize activated B-cell-like diffuse large B-cell lymphoma, primary care physicians follow BRCA1 or BRCA2 mutation carriers, infectious disease doctors treat different hepatitis C virus (HCV)-genotypes, and cardiologists will soon be using organic anion transporting polypeptide 1B1 (SLCO1B1) polymorphisms for tailoring statin therapy (2). Molecular medicine originates from the results of biomedical research aimed at decoding the cellular and molecular mechanisms involved in human health and disease with the ultimate goal of redefining and reclassifying disease states and conditions on a strictly etiological basis. The evolution of technologies targeting the genome (polymerase chain reaction [PCR], comparative genomic hybridization, single nucleotide polymorphism arrays) or the transcriptome (real time quantitative PCR, mRNA, and microRNA expression arrays) facilitate optimized, individualized management of patients. As a result, therapeutic efficacy is maximized by selecting drugs targeting genetically defined patient subpopulations and disease subtypes, while toxicity is circumvented by avoiding treatment in patients predicted to derive no benefit, experience significant toxicity, or have excellent prognosis, regardless of therapy. Thus, the prevailing concept in the emerging framework of molecular medicine is a personalized approach to disease prevention, diagnosis, prognosis, and treatment. Furthermore, the detailed knowledge of molecular pathophysiology has enabled identification of novel strategies—“ novel concepts”—for drug development as exemplified by exploitation of oncogenic addiction (that is, development of epidermal growth factor receptor [EGFR] inhibitors in non-small cell lung cancer) or synthetic lethality (that is, development of poly[ADP-ribose] polymerase [PARP] inhibitors in homologous recombination repair deficient tumors) in cancer therapy (3,4).
The translation of the advances of molecular medicine into clinical practice is not without technical, educational, as well as social-ethical, challenges. Although the application of genomic technologies into discovery of new disease classifications on the basis of novel genetic and epigenetic alterations has been highly successful, application of technologies targeting the transcriptome has yet to contribute much to the day-to-day care of patients. While a large body of literature has described multi-gene expression profiles potentially useful for disease classification, diagnosis, and prognosis (5), only a few of these discoveries have been validated independently and fewer are in the process of further development for potential routine clinical use (as exemplified by use of Oncotype assay in breast cancer therapy) (6). Both the discovery potential and the large-scale validation of microarray findings are limited by the lack of large and adequately annotated frozen tissue repositories and the inherent biases associated with studying single institution-based tissue banks (7).
An emerging challenge for the translation of scientific knowledge into innovative personalized medical therapies stems from the realization that many pathologic conditions (particularly in oncology) contain multiple genetic alterations which lead to dysregulation of diverse signaling pathways and physiologic processes (8,9). A single diagnostic test or a single molecular targeted agent cannot possibly suffice for diseases that are associated with as many as 63 different genetic alterations (8). Only combinatorial treatment strategies encompassing multiple targeted agents and guided by whole genome sequencing or genome-wide gene expression profiling can target such complex and heterogeneous conditions effectively. This would require the development and evaluation of a sizeable armamentarium of molecular therapeutics, which would be administered based on multiple well-validated biomarkers—an admittedly difficult, albeit exciting, task. In many diseases, the distance between elucidating molecular circuitries and developing clinically meaningful interventions is vast and may never be bridged.
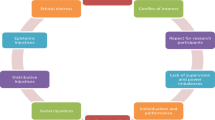
Nevertheless, in as much as mankind has consciously decided to embark on this bold but wonderful journey to decipher the molecular “secrets” of life, sooner or later the riddles of health and disease will be solved. Until that time, the ethical implications of the advances in molecular medicine must not be underestimated. The Health Insurance Portability and Accountability Act (HIPAA) regulation guidelines as well as the Institutional Review Boards (IRB) guarantee the privacy and confidentiality of patient/subject-related information, as well as the protection of human subjects involved in biomedical research. Although the important role of these human subject protection systems is accepted almost universally, there is a growing skepticism regarding the quality and efficiency of these regulatory bodies. In a recent survey (10), several federally funded principal investigators in the United States expressed serious concerns about the IRB system, focusing on the administrative barriers and costs imposed by the ethical review boards as well as the inefficiency, and occasional irrationality, of the IRB review process. There is undoubtedly plenty of room for improvement in several problematic areas of the IRB system, and this is one of most important challenges of contemporary biomedical research.
The ethical conduct of research and the dissemination of its findings extends beyond the researcher/subject interaction. Researchers must carefully balance their responsibility toward their subjects with their need to inform the public about the benefits and risks of genome science. Physicians must practice the art of medicine responsibly in light of discoveries made within the realm of molecular medicine. For the patient, knowledge of genetic predisposition automatically incurs responsibility toward related individuals with whom she/he shares common genomic elements and hence, potentially, common risks of disease. Perhaps more importantly, the era of molecular medicine incurs responsibility toward society as a whole, in the form of disease-preventive adaptations from which both the individual’s health and the society’s wellbeing will benefit. It has been debated whether, in the era of molecular medicine, the right of genetic ignorance should be overshadowed by the obligation of genomic responsibility.

“The successful translation of the advances of biomedical research in everyday clinical practice depends largely upon our ability to train researchers and health professionals in molecular medicine, and to inform and educate the public.”
The responsible conduct of genomic research and the dissemination of information on genetic predisposition to disease extends to the pharmaceutical industry. Corporations must ensure drug safety, availability, and accessibility; provide resources for physicians; increase disease awareness; and lower daily cost of therapy. In the era of personalized medicine, there is a perceived risk by pharmaceutical companies that smaller, targeted patient populations will result in decreased market share and subsequent profit loss. However, the evolution of pharmacogenomics carries the promise of increased drug safety, improved characterization of defined patient populations, and more selective targeting of experimental therapeutics to a disease population. Moreover, experimental therapeutics that once failed clinical trials may demonstrate efficacy in more well-defined patient populations based on genomic information. The resulting reduction in the drug development cycle will lead to decreased drug development costs, improved clinical trial success rates, longer patent life, and greater profits for new drugs (11). Molecular medicine marks a bright future for the pharmaceutical and biotechnology sector, providing that these organizations remain committed to their corporate social responsibilities to sustainable socioeconomic development and improve the quality of life across all members of the society.
The successful translation of the advances of biomedical research in everyday clinical practice depends largely upon our ability to train researchers and health professionals in molecular medicine, and to inform and educate the public. It is the duty of academia to be active in informing and educating both the decision makers and the end users—physicians and patients—about the benefits and promises of molecular medicine. For some specialties in which genetics is already a familiar discipline (for example, pediatrics, oncology, obstetrics, and gynecology), the basic foundation may be in place already, although the speed of genomic advancements may be overwhelming. On the other hand, family practitioners and specialists with more limited exposure to genetics, such as internists and surgeons, will require reeducation about the prospects of molecular medicine.
Decision makers in the political field need to be alerted continuously about the need to prioritize molecular medicine research, with a special focus on collaborative efforts between basic, population, as well as clinical research scientists. The outcome of these interdisciplinary collaborations is a bidirectional approach to molecular medicine research, moving clinical observations to the laboratory environment as well as laboratory discoveries to clinical settings. Funding of these types of collaborations is perhaps best exemplified by the Specialized Programs of Research Excellence (SPOREs) and the Cancer Centers program of the National Cancer Institute (NCI) (12). In these models, investigators from different disciplines work collaboratively to foster research programs that target cancer prevention, diagnosis, and treatment with the ultimate goals of reducing cancer incidence and mortality, and improving survival and quality of life.
However, the task of bringing basic researchers, clinicians, and population scientists together to ensure effective research interactions and research collaborations is not an easy one. Academic medical centers are responsible primarily for establishing appropriate core resources and nurturing collaborative working environments. The dialogue between clinicians and basic scientists must be encouraged institutionally as a way to bridge the gap between hypothesis-driven basic science and problem-focused medical research. Furthermore, the National Institutes of Health (NIH) and private foundations should prioritize grants toward establishment of interdisciplinary research programs around specific diseases and/or disease goals. A major challenge for bringing scientists from different disciplines together is that of evaluation of individual contributions by granting agents as well as academic promotions committees (13). In the era of molecular medicine, it is imperative that a new standard for evaluation of individual contributions in multi-authored papers as well as large interdisciplinary grants needs to be established, as the old paradigm of the “individual scientist who does everything” eventually will cease to exist.
Fostering collaborations and interactions between basic and clinical investigators should be encouraged as early as medical school or during postgraduate residencies and fellowships. Most medical fellowships (that is, hematology, oncology, cardiology, rheumatology, endocrinology, and so on) in leading academic medical centers in the United States have now incorporated mentored research fellowship programs in their curricula, whereby each fellow is required to design and implement a research project prior to completion of their training. The regular interaction with prominent basic scientists from diverse backgrounds and different disciplines enables clinical fellows to think “outside the box,” promotes their ability to conduct interdisciplinary research, and supports their career development and eventual transition into clinical and research independence. This vigorous scientific dialogue also is beneficial for basic scientists who are exposed to the most recent advances in the clinical field while being stimulated by “fresh” inquisitive minds with clinically important research questions.
In the academic setting, expanding the critical mass of the molecular medicine paradigm requires appropriate training of healthcare professionals and biomedical researchers. A very important step toward this has been the establishment of combined MD/PhD programs whereby the vast majority of students receives their PhDs in biomedical laboratory disciplines such as cell biology, biochemistry, immunology, molecular pharmacology, genetics, neuroscience, and biomedical engineering. The success of these programs in producing highly accomplished medical scientists is reflected by the fact that over 80% of MD/PhD program graduates achieve highly sought after positions in academic medicine, government, or industry where molecular medicine research is a central component of their activities (14).
Apart from offering MD/PhD programs, medical schools have the responsibility to provide educational experiences that train medical students to integrate both traditional clinical and modern molecular approaches in clinical scenarios. Curriculum reform remains a top priority issue. In the molecular medicine era, the traditional curriculum whereby comprehensive coverage of biochemistry and other biomedical sciences is provided within the first 2 years while contact with patients and integration of clinical instructional materials is delayed until the last 2 years of medical school can no longer exist. It is important to evolve the traditional biochemistry course by integrating its content across all 4 years of the medical school curriculum (15). Contact with patients and use of clinical scenarios and their resolution as a way to present fundamental biochemical and molecular medicine principles should begin as early as the first 2 years of medical school. Those principles/concepts that need to be covered in greater depth or generalized to multiple systems can be reiterated in the final year of medical school within the context of appropriate course materials (15). This integrative curriculum can provide education in both traditional and molecular disciplines and equip students with the lifelong desire for learning new molecular medicine concepts and keeping up with the rapid proliferation of modern molecular and genetic techniques.
Training in computational biology and bioinformatics is indispensable for physicians and researchers alike to enable the study of complex biological systems in addition to clinical diagnosis, prognosis, and drug selection that will increasingly integrate clinical and genomic data. Although training in computational biology and bioinformatics has yet to be incorporated in the medical school curriculum, there are currently several postgraduate biomedical informatics programs offering master or doctoral degrees for physicians or individuals with advanced degrees in a medically related field. Furthermore, several postgraduate residency and fellowship programs offer rotations or training in computational biology and bioinformatics.
Education of the public about the potential benefits of molecular medicine also remains a top priority. This will appease the common wariness of genome science, which is based either on the belief that such advancement is unnatural and dangerous, or on the fear of discrimination on the basis of genetic testing. Unquestionably, the issue of safeguarding privacy and confidentiality is as important as the education of both the public and healthcare professionals. Furthermore, lay people increasingly will be asked to make decisions about participating in studies that collect genomic data or to make health-related choices and lifestyle modifications based on genomic information about themselves. It is, therefore, important to make people aware of the potential of genomics to identify the genetic basis of disease and of the usefulness of predictive genetic tests as they become available. In this respect, the Internet is a major public resource for information and education. The role of online patient communities deserves special attention as an increasing number of patients obtain health information from online disease-based communities even before visiting a health care professional. These online communities may function not only as a public resource for education about the potential benefits of molecular medicine and the usefulness of predictive genetic tests, but also may accelerate testing of novel therapies as well as recruitment of patients into clinical trials, as exemplified in the case of the rapid recruitment of more than 100 patients in a clinical trial of lithium in amyotrophic lateral sclerosis (16). Finally, the media also can help by presenting the prospects and limitations of molecular medicine accurately and responsibly.
Molecular medicine has revolutionized our understanding and management of several previously incurable diseases. Whether this will translate into optimal handling of patients and global relief of human suffering will probably depend on society as a whole and not solely on the fruits of biomedical research. However, with sound prioritization of goals, a special focus on education and rational allocation of resources, it should be only a matter of time until the novel paradigm of molecular medicine is broadly established as the ultimate standard of care.
Disclosure
We declare that the authors have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
References
Papavassiliou AG. (2001) Clinical practice in the new era. A fusion of molecular biology and classical medicine is transforming the way we look at and treat diseases. EMBO Rep. 2:80–2.
Link E, et al. (2008) SLCO1B1 variants and statin-induced myopathy—a genomewide study. N. Engl. J. Med. 359:789–99.
Kaelin WG, Jr. (2005) The concept of synthetic lethality in the context of anticancer therapy. Nat. Rev. Cancer 5:689–98.
Sharma SV, Bell DW, Settleman J, Haber DA. (2007) Epidermal growth factor receptor mutations in lung cancer. Nat. Rev. Cancer 7:169–81.
Quackenbush J. (2006) Microarray analysis and tumor classification. N. Engl. J. Med. 354:2463–72.
Paik S, et al. (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 351:2817–26.
Konstantinopoulos PA, Spentzos D, Cannistra SA. (2008) Gene-expression profiling in epithelial ovarian cancer. Nat. Clin. Pract. Oncol. 5:577–87.
Jones S, et al. (2008) Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321:1801–6.
Parsons DW, et al. (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–12.
Whitney SN, et al. (2008) Principal investigator views of the IRB system. Int. J. Med. Sci. 5:68–72.
Breckenridge A, et al. (2004) Pharmacogenetics: ethical problems and solutions. Nat. Rev. Genet. 5:676–80.
Nathan D, Benz EJ. (2001) Comprehensive cancer centres and the war on cancer. Nat. Rev. Cancer 1:240–5.
Nathan DG. (2002) Careers in translational clinical research-historical perspectives, future challenges. JAMA 287:2424–7.
Schwartz P, Gaulton GN. (1999) Addressing the needs of basic and clinical research: analysis of graduates of the University of Pennsylvania MD-PhD program. JAMA 281:96–7.
Smith HC, Hundert EM. (1999) Integrating molecular medicine into the medical school curriculum. Mol. Med. Today 5:102–3.
Fornai F, et al. (2008) Lithium delays progression of amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. U. S. A. 105:2052–7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Konstantinopoulos, P.A., Karamouzis, M.V. & Papavassiliou, A.G. Educational and Social-Ethical Issues in the Pursuit of Molecular Medicine. Mol Med 15, 60–63 (2009). https://doi.org/10.2119/molmed.2008.00120
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/molmed.2008.00120