Abstract
Tyroserleutide (YSL) is an active, low-molecular-weight polypeptide, comprised of three amino acids, that has shown antitumor effects on human hepatocarcinoma BEL-7402 in vitro and in vivo. In this study, we evaluated the inhibition of YSL on invasion and adhesion of the mouse B16-F10 melanoma cell line by injecting B16-F10 cells into the tail veins of C57BL/6 mice to establish an experimental lung metastasis model. YSL inhibited B16-F10 cell metastasis to lung, reducing the number and area of metastasis lesions. When we treated B16-F10 cells with YSL (0.01, 0.1, 1, 10, or 100 µg/mL) in vitro, we found that YSL inhibited the proliferation of B16-F10 cells with a 28.11% rate of inhibition. YSL significantly decreased the adhesiveness of B16-F10 cells to Matrigel with a 29.15% inhibition rate; YSL also significantly inhibited the invasion of B16-F10 cells, producing an inhibition of 35.31%. By analyses with Western blot and real-time RT-PCR, we found that YSL markedly inhibited the expression of ICAM-1 in B16-F10 cells. These data suggest that YSL inhibits the growth, invasion, and adhesion of B16-F10 cells.
Similar content being viewed by others
Introduction
Cancer continues to represent the largest cause of mortality in the world and claims more than six million lives every year. Metastasis, rather than primary tumor growth, is the most fearsome aspect of cancer and the common cause of cancer deaths (1). It is crucial to control the invasion and metastasis of tumors in the treatment for cancer. GIP (growth inhibitory peptide) has been reported to inhibit the growth, adhesion, and metastasis of some tumors (2). RGD (Arg-Gly-Asp) and YIGSR (Tyr-Ile-Gly-Ser-Arg) also showed the same effects on mouse melanoma (3,4).
Tyrosyl-seryl-leucine (tyroserleutide, or YSL, as approved by the Chinese pharmacopoeia committee) is a tripeptide compound that was developed in China. It consists of three natural amino acids, L-tyrosine, L-serine, and L-leucine, with a corresponding chemical structure C18H27N3O6. The molecular weight of YSL is 381.42. In our previous study, YSL exhibited a significant antitumor effect on human hepatocarcinoma BEL-7402 cells transplanted into nude mice, with a 64.17% rate of inhibition at 160 µg/kg per day (5). In this study, we systematically observed the effect of YSL on metastasis of mouse melanoma B16-F10 cells. B16-F10 is a highly metastatic melanoma cell line (6) that has been used in many studies on the processes of tumor metastasis. By establishing an experimental lung metastasis model of B16-F10 in C57BL/6 mice, we observed the effect of YSL on lung metastasis. A B16-F10 culture system in vitro was used to observe the influence of YSL on proliferation, adhesion, and invasion of tumor cells. Because ICAM-1 (CD54), a cell adhesion factor of the immunoglobulin superfamily, has been closely associated with the processes of invasion and metastasis of the tumor (7), we further studied the effect of YSL on mRNA and protein expression of ICAM-1 in B16-F10 cells.
Materials and Methods
Drugs
Shenzhen Kangzhe Pharmaceutical, China, manufactured tyroserleutide. Saline was from China Otsuka Pharmaceutical, China.
Cell Culture
The highly metastatic cell line, murine melanoma B16-F10, was from the Institute of Material Medica, Chinese Academy of Medical Sciences. The cells, which were routinely examined and found to be free of mycoplasma contamination, were cultured in RPMI 1640 (Gibco Invitrogen) supplemented with 10% FBS (Hyclone Laboratories) in a humidified atmosphere of 5% CO2 at 37°C.
Animals
Healthy female C57BL/6 mice (4 to 5 weeks old, 18 to 22 g) were from the Academy of Military Medical Sciences (Beijing, China). The animals were maintained at our university under specific pathogen-free (SPF) conditions according to the guidelines of the Chinese Association for Laboratory Animal Care, using a laminar air-flow rack, and had continuous access to sterilized pelleted food and autoclaved water with a controlled light/dark cycle, temperature at 20 to 25°C, and 50% to 60% relative humidity with 15 fresh air changes per hour. All studies were carried out with approved institutional experimental animal care and use protocols. Experiments commenced after one week of acclimatization.
Experimental Lung Metastasis Model
To establish the lung metastasis model (8), 2 × 105 B16-F10 cells were injected into the tail veins of C57BL/6 mice. Mice were randomly divided into four groups of 10 and received daily injections of saline only (0.2 mL/day) or YSL (80, 160, and 320 µg/kg/day) from day 2. YSL, a whitish, lyophilized powder, was dissolved in saline to the requested concentration. To determine how much YSL to dissolve, we took the average body weight of mice as 20 g; each mouse received 0.2 mL YSL solution or saline intraperitoneally. The animals were euthanized on day 21. The lungs were removed and fixed in Bouin’s solution. The numbers of lung metastasis lesions were counted under a dissecting microscope. The following formula was used to calculate the metastasis inhibition: rate of inhibition (%) = (1 — number of metastasis lesions in treatment group/number of metastasis lesions in saline group) × 100. Formalin-fixed, paraffin-embedded sections of each lung tissue sample were stained routinely with hematoxylin and eosin (HE). Histopathology examination was completed using Olympus microscopy (9).
In Vitro Melanoma Cell Proliferation Assays
B16-F10 cells in exponential growth phase were suspended in RPMI 1640 containing 10% FBS and cultured in flatbottomed, 96-well plates (2 × 104/well) for 24 h at 37°C. Then supernatants were removed, and YSL (diluted in RPMI 1640) was added (100 µL/well). The final concentrations of the drug were 0.01, 0.1, 1, 10, and 100 µg/mL, and plain RPMI 1640 was the negative control. Plates were incubated in a humidified incubator in 5% CO2 at 37°C for 24 or 48 h. Supernatants were discarded after centrifugation. MTT (0.5 mg/mL) was added to the plates at 100 µL/well, and incubation continued for 4 h. After centrifugation, supernatants were again discarded, and 40 mM HCl-isopropanol (100 µL/well) was added to the cell pellets, which were shaken for 3 min. An ELISA reader (Bio-Rad, model 550) was used to measure the OD570 (A value) of each well referenced at 630 nm. The following formula was used to calculate the inhibition of cell growth: rate of inhibition (%) = (1 — mean A value of treatment group/mean A value of control) × 100.
Melanoma Cell Adhesion Assay
For the melanoma cell adhesion assay (10), matrigel (BD Biosciences; 200 µg/mL) was added to a flat-bottomed, 96-well plate at 25 µL/well. The plate was incubated at 37°C for 1 h, washed with PBS, and dried at room temperature and 2% BSA (Sigma) was added to the plate (20 µL/well). The plate was incubated at 37°C for 1 h, washed with PBS, and dried at room temperature for 1 h. B16-F10 cells with YSL (0.01, 0.1, 1, 10, or 100 µg/mL) and RPMI 1640 (negative control) were collected by digestion with trypsin, and the cells (5 × 105/mL) were suspended in 0.1% BSA-RPMI 1640 and added to wells coated with Matrigel (100 µL/well). Incubation continued for 1 h. No adherent cells were removed by aspiration and 3 additional washes with PBS. The A values of all wells were measured by the MTT method at 570 nm. The following formula was used to calculate the inhibition of cell adhesion: rate of inhibition (%) = (1 — mean A value of treatment group/mean A value of control) × 100.
Invasion Assay
The invasive activity of the B16-F10 cells was assayed in a transwell cellculture chamber (Costar) (11). The lower surfaces, polyvinylpyrrolidone-free polycarbonate filters with a pore size of 8.0 µm, were coated with 5 µg fibronectin in a volume of 25 mL and dried at room temperature. Reconstituted basement membrane Matrigel was applied to the upper surface of the filter (5 µg/filter) to form a matrix barrier. Coated filters were washed with PBS and dried immediately before use. Tumor cells (already treated with 0.01, 0.1, 1, 10, 100 µg/mL YSL or RPMI 1640 for 24, 48, or 72 h) were harvested with RPMI 1640 supplemented with 0.1% BSA. Tumor cell suspensions (2 × 106 cells/mL, 100 mL) were added to the upper compartment of the chamber and incubated for 4 h. After incubation, the filters were harvested, fixed with methanol, and stained with HE. Tumor cells on the upper surfaces of the filters were removed by wiping with cotton swabs. Cells that had passed through the Matrigel and filter to various areas on the lower surface were counted under a microscope in 5 fields at ×100 magnification. Each assay was performed in triplicate and repeated at least 3 times. The following formula was used to calculate the inhibition of cell invasion: rate of inhibition (%) = (1 — number of invading cells of the treatment group/number of invading cells of control group) × 100.
RNA Extraction and Real-Time PCR (12)
To prepare for PCR (12), B16-F10 cells were incubated with YSL (0.01 or 0.1 µg/mL) or RPMI 1640 (negative control) for 48 h. Total RNA was extracted from cells using Trizol Reagent (MRC) according to the manufacturer’s protocol. First-strand cDNA synthesis was performed using 2 µg total RNA with M-MLV Reverse Transcriptase (Promega) in recommended conditions, using 500 ng oligo(dT) 18. Quantitative PCR to amplify the ICAM-1 gene was performed on an ABI Prism 7500 Sequence Detection System (Applied Biosystems) using SYBR Green Real-time Master Mix (Toyobo). The sequences of forward and backward primers of ICAM-1 were 5′-CGCACAGAACTGGATCTCAGGC-3′ and 5′-GCTTCAGAGGCAGGAAACAGGC-3′, respectively. Amplification conditions were as follows: 95°C for 1 min, then 45 cycles of 95°C for 15 s, 59°C for 15 s, and 72°C for 45 s. β-Actin was used as an active and endogenous reference to correct differences for total RNA added to reaction and to compensate for different levels of inhibition during reverse transcription of RNA and during PCR. The sequences of forward and backward primers of β-actin were 5′-GGCTGTATTCCCCTCCATCG-3′ and 5′-CCAGTTGGTAACAATGCCATGT-3′, respectively. Melting curves were calculated using Dissociation Curves software (Applied Biosystems) to ensure only a single product was amplified, and samples were loaded on a 2% agarose gel to confirm specificity. The threshold cycle (CT) indicates the fractional cycle number at which the amount of amplified target gene reaches a fixed threshold. The relative amount of ICAM-1 was calculated using the 2−ΔCT equation, where ΔCT = CT of target gene — CT of housekeeping gene (13).
Western Blot
For Western blot (14), after B16-F10 cells were treated with YSL (0.01 or 0.1 µg/mL) or RPMI 1640 (negative control) for 48 h, total lysate protein was prepared in radioimmunoprecipitation assay buffer (RIPA). Samples were mixed with sample buffer, boiled for 10 min, separated by 0.1% SDS, 12% polyacrylamide gel electrophoresis under denaturing conditions, and electroblotted to Immobilon-P membranes (Millipore). The membranes were blocked with TBS (5% nonfat dry milk, 0.1% Tween 20 in a 50 mM Tris-HCl buffer, 150 mM NaCl, pH 7.5) overnight at 4°C. The membranes were incubated with anti-ICAM-1 antibody (R&D) or anti-β-actin antibody (Sigma) for 2 h at room temperature. After 3 washes in TBS/0.1% Tween 20, membranes were probed with a secondary goat anti-rat (Santa Cruz) or goat anti-mouse (KPL) antibody, each conjugated to horseradish peroxidase (HRP). After washing 3 times with TBS and 0.1% Tween 20, immune complexes were visualized with a SuperSignal West pico Trial Kit (Pierce). The membrane was reprobed with β-actin to correct for small differences in loading. The resulting bands on film were quantitated by computer-assisted video densitometry. The amount of protein under control conditions was assigned a relative value of 100%.
Statistical Analysis
Data are expressed as mean ± SD. Statistical significance was tested using one-way analysis of variance followed by the Student-Newman-Keuls test. Statistical significance was set at P < 0.05.
Results
Effect of YSL on the Experimental Lung Metastasis Model of B16-F10 Cells
Experimental metastasis refers to the injection of tumor cells directly into the systemic circulation. The most common site of tumor cell injection employed for experimental metastasis models is the lateral tail vein in mice. Tail vein injection results primarily in pulmonary metastases. This model simulates all the processes that occur after tumor cells enter the blood circulation (15). According to the effect of YSL on the growth of human hepatocarcinoma Bel-7402 transplanted into nude mice (5), we chose doses from 80 to 320 µg/kg per day. In this study, YSL (160~320 µg/kg per day) significantly inhibited B16-F10 cell lung metastasis. There was a significant difference between the numbers of metastasis lesions in the experimental and saline-treated groups (P < 0.05). Dosages of 160 and 320 µg/kg/d produced rates of inhibition of 55.6% and 62.1%, respectively (Figure 1a). Pathologic results in the saline group showed lung metastatic lesions mostly surrounding the blood vessel, trachea vessel, or pleura, dispersing in whole lung tissue (Figure 1b–d). The area of metastatic lesions in the YSL-treated group was smaller than in the saline-treated group (data not shown), and more necrosis in metastatic lesions was observed in the YSL group. YSL at 80 µg/kg per day reduced the number of metastasis lesions, showing a trend to inhibit the B16-F10 growth and lung metastasis, but there was no significant difference between YSL and saline groups. In addition, on day 21, the control mice showed more weight loss, decrease in activity, frequent clustering together, and lackluster hair. Similar changes were observed in the YSL group, but in general, their condition was better than that of the saline group.
YSL effects on the experimental lung metastasis model of B16-F10 cells. C57/BL6 mice (10 mice/group) received intravenous injection of B16-F10 melanoma cells (2 × 105 cells/mouse) and killed 20 d later. The lungs were collected and fixed in Bouin’s solution. The numbers of lung metastatic lesions were counted under a dissecting microscope. The results are expressed as the means ± SD. (a) Impact of YSL on the number of lung metastasis lesions of B16-F10 cells. *P< 0.05 vs. control (saline) group. Formalin-fixed, paraffin-embedded sections of each lung tissue were stained routinely with HE. Histopathology examination was completed using Olympus microscopy. (b) YSL 320 µg/kg per day: HE, ×40, nidulant lung metastatic lesions surrounded the pleura, blood vessel, or trachea vessel; (c) YSL 160 µg/kg/d: HE, ×40, nidulant lung metastatic lesions near the pleura; (d) saline: HE, ×40, nidulant lung metastatic lesions dispersed in whole lung tissue.
Cytotoxic Function of YSL against B16-F10 Cells In Vitro
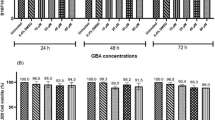
From the study of the experimental lung metastasis model of B16-F10, we found that YSL administered by celiac injection reduced the number and the area of metastatic lung lesions. The cytotoxic function of YSL against B16-F10 cells was assayed with MTT (16) in vitro. After treating B16-F10 cells with YSL (0.01, 0.1, 1, 10, or 100 µg/mL) for 24, 48, or 72 h, the dose-effect curve of YSL on B16-F10 cell proliferation had a bell shape. After treatment with YSL (0.01 or 0.1 µg/mL for 24 h or 0.01, 0.1, or 1 µg/mL for 48 or 72 h), the proliferation of B16-F10 cells was inhibited compared with the negative control (P < 0.05) (Figure 2).
Inhibitory effect of YSL on B16-F10 cells in vitro. B16-F10 cells had been treated with YSL (0.01, 0.1, 1, 10, or 100 µg/mL) in vitro for 24, 48, or 72 h. Cytotoxic function was assayed with MTT. The results are expressed as the rate of growth inhibition: rate of inhibition (%) = (1 — mean A value of treatment group/mean A value of control) × 100. *P < 0.05 compared with control group.
Inhibitory Effect of YSL on B16-F10 Cell Adhesion to Matrigel
The adhesion of tumor cells includes the conjunction of tumor cells themselves as well as the adhesion of tumor cells and other type of cells. Adhesive ability of metastatic tumor cells is a critical factor in extravasations and formation of new tumor foci (1). Matrigel is a solubilized basement membrane preparation extracted from EHS mouse sarcoma, a tumor rich in ECM proteins. Its major component is laminin, followed by collagen IV, heparan sulfate proteoglycans, and entactin. Matrigel can produce biologically active matrix material resembling the mammalian cellular basement membrane and provide a physiologically relevant environment for studies of migration or invasion (17). After treating B16-F10 cells with various doses of YSL (0.01~100 µg/mL) for 24, 48, or 72 h, the dose effect of YSL on B16-F10 cell adhesion to Matrigel yielded a bell-shape curve. After treating B16-F10 cells with YSL for 24 h, only 0.1 µg/mL YSL inhibited the adhesion of B16-F10 cells to Matrigel. After treatment for 48 h, all doses of YSL (0.01~100 µg/mL) inhibited the adhesion of B16-F10 cells to Matrigel. When incubated with B16-F10 for 72 h, YSL (0.01, 0.1, and 1 µg/mL) inhibited the adhesion of B16-F10 cells to Matrigel (P < 0.05) (Figure 3).
Inhibitory effect of YSL on B16-F10 cell adhesion to Matrigel. Ninety-six-well cell culture plates were coated with 25 mL Matrigel (200 µg/mL) and incubated at 37°C for 1 h and then washed with PBS. The coated wells were overlaid with 1% BSA for 1 h, washed with PBS, and dried at room temperature. Cells treated with YSL for 24, 48, or 72 h were applied to individual wells coated with Matrigel at 5 × 105/mL and incubated for 1 h at 37°C in 5% CO2. The A values of all wells were measured by the MTT method at 570 nm. The results are expressed as the inhibition of cell adhesion of 6 experiments: rate of inhibition (%) = (1 — mean A value of treatment group/mean A value of control) × 100. *P < 0.05 compared with control group.
Inhibitory Effect of YSL on B16-F10 Invasion
Tissue invasion by tumor cells involves their migration across basement membranes through activation of extracellular matrix degradation and cell motility mechanisms. Tumor invasion into the extracellular matrix plays an important role in tumor metastasis (18). In this study, B16-F10 cells induced by FN passed through polycarbonate membrane coated with Matrigel in a transwell cell-culture chamber, in which the number of invading cells reflected the invasive capacity of the cells. The B16-F10 cells were treated in advance with various doses of YSL (0.01~100 µg/mL) for 24, 48, or 72 h before they were added into the transwell chambers. The dose-effect curve of YSL on B16-F10 cell invasion was also bell shaped. The results showed that YSL (0.01, 0.1, and 1 µg/mL) significantly inhibited the invasion of B16-F10 cells that had been treated in advance for 48 h in vitro (P < 0.05) (Figure 4).
Inhibitory effect of YSL on B16-F10 cell invasion. Polyvinylpyrrolidone-free polycarbonate filters with a pore size of 8 µm were coated with fibronectin on the lower surface. Reconstituted basement membrane Matrigel was added to the upper surface of the filter to form a matrix barrier. B16-F10 cells treated with various doses of YSL (0.01~100 µg/mL) and RPMI 1640 (control) for 24, 48, or 72 h were incubated in the upper compartment of a transwell culture chamber for 4 h. The numbers of invasion cells were determined as described previously. The result was expressed as follows: rate of inhibition (%) = (1 — number of invading cells of the treatment group/number of invading cells of control group) × 100. (A) Effect of YSL on the number of invading B16-F10 cells. *P< 0.05 compared with control group. (B) YSL 0.01 µg/mL, HE, ×200; (C) YSL 0.1 µg/mL, HE, ×200; (D) YSL 1 µg/mL, HE, ×200; (E) control group, HE, ×200.
Inhibitory Effect of YSL on the mRNA Expression of ICAM-1 Analyzed by Real-Time PCR
Adhesion molecules are involved in intracellular signaling in various physiological and pathological processes including metastasis and growth of tumor cells. ICAM-1 (CD54) belongs to the immunoglobulin superfamily of adhesion molecules. It can reflect the potential ability of tumor invasion and metastasis to a certain extent (19,20). Real-time quantitative reverse-transcription PCR was used to detect the expression of ICAM-1 mRNA in B16-F10 cells in this study. After treatment with YSL (0.01 or 0.1 µg/mL) or RPMI 1640 (control) for 48 h, RNA was extracted from B16-F10 cells and through reverse transcription and real-time PCR to evaluate ICAM-1 gene expression. The results showed that YSL (0.01 and 0.1 µg/mL) significantly decreases ICAM-1 mRNA expression in B16-F10 cells (P < 0.05) (Figure 5).
Inhibitory effect of YSL on mRNA expression of ICAM-1. After B16-F10 cells were treated with YSL (0.01 or 0.1 µg/mL) or RPMI 1640 (control) for 48 h, mRNA was extracted and reverse transcribed to cDNA. The cDNA was subjected to realtime quantitative PCR using gene-specific primers for ICAM-1, and β-actin was used as reference. The relative amount of ICAM-1 was calculated using the 2−ΔCT equation. n = 3, mean ratio ± SD; *P< 0.05 compared with control group.
Inhibitory Effect of YSL on the Protein Expression of ICAM-1
A marked and significant decrease in mRNA expression of ICAM-1 was observed in YSL-treated B16-F10 cells by real-time quantitative RT-PCR. Western blot analysis of ICAM-1 in B16-F10 cells showed that YSL (0.01 or 0.1 µg/mL for 48 h) decreased the expression of ICAM-1 (Figure 6).
Inhibitory effect of YSL on protein expression of ICAM-1. (A) After B16-F10 cells were treated with YSL (0.01 or 0.1 µg/mL) or RPMI 1640 (control) for 48 h, protein was extracted and assessed by Western blot. (B) Representative figure shows analysis of ICAM-1 expression. n = 3, mean ratio ± SD; *P < 0.05 compared with control group.)
Discussion
Tumor metastasis is a complex process. Tumor cells leave the site of the primary tumor and pass through the tumor basement membrane, enter the circulation, and eventually arrest at distant organs. At this distant site, the cell leaves the circulation or proliferates within the vessel, survives in the novel microenvironment of a foreign tissue site, proliferates, creates new blood vessels (angiogenesis), coopts existing blood vessels or grows within an existing vessel, and then successfully grows into a clinically relevant metastasis lesion. Because of the complexity of the metastatic process and the changing microenvironmental cues and interactions that a disseminated cell experiences, the development of such an in vitro assay system is unlikely in the near future. In vivo models, therefore, must continue to be an important workhorse in antimetastasis drug screening. Some potent anti-angiogenesis drugs, such as VEGF receptor inhibitor Z24 (21) and endostatin (22), have been found through in vivo modeling. Our early study revealed that the highest rate of inhibition of YSL on human hepatocarcinoma BEL-7402 cells in vitro was merely 36.29%, but the result of animal experiments in vivo showed that YSL at 160 and 320 µg/kg per day inhibited BEL-7402 growth in nude mice by 64.17% and 59.19% (5). According to the effect of YSL on the growth of BEL-7402 transplanted into nude mice, doses ranging from 80 to 320 µg/kg per day were used in experimental metastasis models in vivo. We established the experimental lung metastasis model to observe the inhibition of YSL on lung metastasis of mouse melanoma B16-F10. The results showed that YSL (320 and 160 µg/kg per day) administered by celiac injection significantly reduced the number and area of metastasis lung lesions, which mainly emerge at membrana pleuralis or hilum of lung, inhibiting incursions of tumor into interalveolar septum and bronchi. Moreover, YSL decreased the formation of tumor embolus in blood vessels to inhibit blood-borne metastasis of tumor cells.
Tumor metastasis begins from invasion. Tumor invasion includes the penetration of blood vessels and lymphatic vessels, and the invasion of target organs by tumor cells and plays an important role in tumor metastasis (18). Liotta et al. (23) proposed a three-step hypothesis describing the sequence of biochemical events during tumor cell invasion. The first step is tumor cell attachment via cell-surface receptors that specially bind to the matrix components such as lamin (for BM) and fibronectin (for the stroma). The anchored tumor cell next secretes hydrolytic enzymes (or induces host cells to secrete enzymes) that can locally degrade the matrix (including degradation of the attachment components). Matrix lysis most probably occurs in a highly localized region close to the tumor cell surface. The third step is tumor cell migration to the matrix region modified by proteolysis. Continued invasion of the matrix may take place by cyclic repetition of these three steps. From the effects of YSL on adhesion, invasion of tumor cells, and its cytotoxic function, we analyzed the mechanism of YSL inhibiting tumor metastasis stepwise in vitro, each experiment representing a certain aspect of YSL anticancer function. The beginning dose of YSL was chosen as 100 µg/mL, then diluted by ten times down to 0.01 µg/mL to observe every dose of YSL effect on B16-F10 metastasis. The result indicated that all doses of YSL significantly decreased the adhesiveness of B16-F10 cells to Matrigel, implying that the adhesiveness ability of tumor cells to the extracellular matrix of secondary neoplasm was decreased by YSL. Moreover, in an invasion assay, B16-F10 cells were induced by FN to pass through polycarbonate membrane coated with Matrigel in a transwell cell-culture chamber, in which the number of invading cells reflected the invasive capacity of the cells. The result showed that YSL (0.01, 0.1, and 1 µg/mL) significantly inhibited the invasiveness of B16-F10 cells, so we infer that tumor cells treated with YSL have no potential to metastasize due to their low invasive capacity, even should they detach from the primary neoplasm. Through cell proliferation assays in vitro, we found that after treatment with YSL (0.01 or 0.1 µg/mL for 24 h or 0.01, 0.1, or 1 µg/mL for 48 or 72 h), the proliferation of B16-F10 cells was inhibited. With this result, along with our finding that in vivo both the number and area of metastasis lung lesions was reduced, we presume that the antimetastasis mechanism of YSL may involve direct inhibition of tumor cell growth.
Finally, we evaluated the effect of YSL on the expression of ICAM-1 (CD54), a member of the immunoglobulin superfamily of adhesion molecules. ICAM-1 mediates the interaction of tumor cells with the extracellular matrix, blood cells, and the cells of target organs and is closely associated with the processes of invasion and metastasis. It has been reported that the degree of malignancy is directly proportional to the expression of ICAM-1, as well as the dangerous metastasis of tumor (24). The above experiments showed that the optimum effective concentrations of YSL on B16-F10 were 0.01 and 0.1 µg/mL, so we chose these 2 doses to analysis the effect of YSL on ICAM-1 expression. In real-time quantitative reverse-transcription PCR and Western blot assays, we found that YSL (0.01 and 0.1 µg/mL) inhibited mRNA and protein expression of ICAM-1 in B16-F10 cells. Decreased expression of ICAM-1 can reduce the adhesive ability of B16-F10 cells to extracellular matrix, thereby inhibiting the invasion and metastasis of tumor cells.
Interestingly, we found that in vivo results of inhibition of YSL were better than in vitro observations. The possible mechanisms may result from the following presumptions: (1) Our previous studies showed that YSL-induced tumor inhibition was associated with alteration of several cell functions in vivo. We think that in vitro observations were too simple to reveal the complete antitumor effects of YSL. (2) This could be due to the difference in doses between in vitro and vivo experiments. According to our many preliminary studies, we selected 80 to 320 µg/kg per day as the dosage of YSL in animal experiments, which normally were deduced from the effective dosage in vitro. Therefore, the difference in doses may result in different results. (3) We presume the different administration periods may be for one of the following reasons. The mice with lung metastasis were treated with YSL for 20 days, whereas in the in vitro model, YSL was given for only 24 to 72 h. Therefore, we think that the longer period of administration of YSL may generate better therapeutic efficacy. Moreover, YSL inhibited the adhesion, invasion, and proliferation of tumor cells in vitro with bell-shaped dose-effect curves.
The common dose-effect curve can be S-shaped, bell-shaped, or U-shaped. There are many reports on the bell-shaped dose-effect curve, such as 5-HT3 receptor inhibitor-tropisetron (25) and allicin (the main organic allyl sulfur component in garlic) (26). Curnis et al. (27) showed that targeted delivery of low doses of IFN-γ to CD13, a marker of angiogenic vessels, could delay tumor growth in two murine models that respond poorly to IFN-γ. The dose-response curve was bell-shaped; maximal effects were induced with a dose of 0.005 µg/kg. Barnard and Gurevich (28) found that low doses of BAS (biologically active substance) stimulated cellular response in vitro, whereas high doses inhibited it. Unlike cytotoxic drugs, most anti-angiogenesis drugs are not typically dose dependent. On the contrary, some kinds of anti-angiogenesis show a higher therapeutic index at lower doses. At high doses, it might be antagonism that results in the decrease, even loss, of biological activity. In addition, dose-effect curves of many peptides such as pyroGlu-His-GlyOH (29) and Sandostatin (octreotide) are bell-shaped. Growth inhibitory tripeptide, pyroGlu-His-GlyOH colon mitosis inhibitor (CMI), was isolated from water extracts of mouse intestine. It was demonstrated in two different experiments that intraperitoneal injections of CMI, daily or three times a week, could inhibit the long-term subcutaneous growth of HT-29 cells in athymic mice. The growth-inhibitory effect was most pronounced initially with approximately 50% to 90% growth inhibition, and declined terminally with approximately 40% to 60% growth inhibition. A bell-shaped dose-response curve, with maximal effect at 1 pmol/animal/injection, was seen (30). In addition, Sandostatin (octreotide) can also influence the proliferation of human breast cancer cells (MCF-7)—namely, in an inhibitory fashion. A bell-shaped dose-effect curve was observed with the maximal inhibition of tumor cell growth at a sharply defined amount of Sandostatin (10 nM) (30). As with these drugs, the dose-effect curve of YSL is also bell-shaped, but the mechanisms are not very clear. We presume that the mechanisms may be (1) Desensitization of receptors, i.e., receptors with prolonged or repeated exposure to certain drugs, which often leads to the decrease of receptor response to outside formation. This phenomenon usually is observed in conditions of phosphorylation or ingression of receptors. (2) Combination with different types of receptors leads to the opposite pharmacological role. In low concentrations, the drug plays a certain role through only combining with one receptor; in high concentrations, it plays an opposing role through combining with a different receptor. This results in a bell-shaped dose-effect curve. (3) Change of effector’s molecule conformation or existence of a negative back loop. More possible mechanisms of YSL need to be explored further.
References
Pan Y, Song QL, Lin YH, Lu N, Yu HM, Li XJ. (2005) GLB prevents tumor metastasis of Lewis lung carcinoma by inhibiting tumor adhesion actions. Acta Pharmacol. Sin. 26:881–6.
Muehlemann M, Miller KD, Dauphinee M, Mizejewski GJ. (2005) Review of growth inhibitory peptide as a biotherapeutic agent for tumor growth, adhesion, and metastasis. Cancer Metastasis Rev. 24:441–67.
Yamamoto Y, Tsutsumi Y, Mayumi T. (2002) Molecular design of bioconjugated cell adhesion pep-tide with a water-soluble polymeric modifier for enhancement of antimetastatic effect. Curr. Drug Targets 3:123–30.
Kato R et al. (2002) A new type of antimetastatic peptide derived from fibronectin. Clin. Cancer Res. 8:2455–62.
Lu R et al. (2005) Experimental study of the inhibition of human hepatocarcinoma Bel7402 cells by the tripeptide tyroserleutide (YSL). Cancer Chemother. Pharmacol. 57:1–9.
Kawasaki M, Maeda T, Hanasawa K, Ohkubo I, Tani T. (2003) Effect of His-Gly-Lys motif derived from domain 5 of high molecular weight kininogen on suppression of cancer metastasis both in vitro and in vivo. J. Biol. Chem. 278:49301–7.
Okegawa T, Pong RC, Li Y, Hsieh JT. (2004) The role of cell adhesion molecule in cancer progression and its application in cancer therapy Acta Biochim. Pol. 445–57.
Mummert ME, Mummert DI, Ellinger L, Takashima A. (2003) Functional roles of hyaluronan in B16-F10 melanoma growth and experimental metastasis in mice. Mol. Cancer Ther. 2:295–300.
McGary EC et al. (2003) Afully human antimelanoma cellular adhesion molecule/MUC18 antibody inhibits spontaneous pulmonary metastasis of osteosarcoma cells in vivo. Clin. Cancer Res. 9:6560–6.
Sullivan RM, Stone M, Marshall JF, Uberall F, Rotenberg SA. (2000) Photo-induced inactivation of protein kinase calpha by dequalinium inhibits motility of murine melanoma cells. Mol. Pharmacol. 58:729–37.
Zhang XM, Huang SP, Xu Q. (2004) Quercetin inhibits the invasion of murine melanoma B16-BL6 cells by decreasing pro-MMP-9 via the PKC pathway. Cancer Chemother. Pharmacol. 53:82–8.
Dussault AA, Pouliot M. (2006) Rapid and simple comparison of messenger RNA levels using real-time PCR. Biol. Proc. Online 8:1–10.
Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-Δ Δ CT method. Methods 25:402–8.
Usui T, Ishida S, Yamashiro K, et al. (2004) VEGF164 (165) as the pathological isoform: differential leukocyte and endothelial responses through VEGFR1 and VEGFR2. Invest. Ophthalmol. Vis. Sci. 45:368–74.
Yamaguchi H et al. (2003) Sphingosine-1-phosphate receptor subtype-specific positive and negative regulation of Rac and haematogenous metastasis of melanoma cells. Biochem. J. 374:715–22.
Theiszova M, Jantova S, Dragunova J, Grznarova P, Palou M. (2005) Comparison the cytotoxicity of hydroxyapatite measured by direct cell counting and MTT test in murine fibroblast NIH-3T3 cells. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 149:393–6.
Zheng HQ, Liu DY. (2003) [Anti-invasive and anti-metastatic effect of ampelopsin on melanoma]. Ai Zheng 22:363–7.
Ogasawara M, Matsunaga T, Takahashi S, Saiki I, Suzuki H. (2002) Anti-invasive and metastatic activities of evodiamine. Biol. Pharm. Bull. 25:1491–3.
Tachimori A et al. (2005) Up regulation of ICAM-1 gene expression inhibits tumor growth and liver metastasis in colorectal carcinoma. Eur. J. Cancer 41:1802–10.
Yashiro M, Sunami T, Hirakawa K. (2005) CD54 expression is predictive for lymphatic spread in human gastric carcinoma. Dig. Dis. Sci. 50:2224–30.
Lu H et al. (2005) Angiogenesis inhibitor Z24 induces endothelial cell apoptosis and suppresses tumor growth and metastasis. J. Pharmacol. Sci. 97:533–40.
Ren B, Hoti N, Rabasseda X, Wang YZ, Wu M. (2003) The antiangiogenic and therapeutic implications of endostatin. Methods Find Exp. Clin. Pharmacol. 25:215–24.
Liotta LA et al. (1988) Biochemical mechanisms of tumor invasion and metastases. Prog. Clin. Biol. Res. 256:3–16.
Wang Y et al. (2004) Inhibitors of 5-lipoxygenase inhibit expression of intercellular adhesion molecule-1 in human melanoma cells. Acta Pharmacol. Sin. 25:672–7.
Stratz T, Kees F, Muller W. (2003) Do cytochrome enzymes influence the therapeutic effect of tropisetron in fibromyalgia? Drugs Exp. Clin. Res. 29:91–4.
Patya M et al. (2004) Allicin stimulates lymphocytes and elicits an antitumor effect: a possible role of p21ras. Int. Immunol. 16:275–81.
Curnis F, Gasparri A, Sacchi A, Cattaneo A, Magni F, Corti A. (2005) Targeted delivery of IFNgamma to tumor vessels uncouples antitumor from counterregulatory mechanisms. Cancer Res. 65:2906–13.
Barnard R, Gurevich KG. (2005) In vitro bioassay as a predictor of in vivo response. Theor. Biol. Med. Model 2:3.
Paulsen JE. (1993) The synthetic colon peptide pyroGlu-His-GlyOH inhibits growth of human colon carcinoma cells (HT-29) transplanted subcutaneously into athymic mice. Carcinogenesis 14:1719–21.
Setyono-Han B, Henkelman MS, Foekens JA, Klijn GM. (1987) Direct inhibitory effects of somatostatin (analogues) on the growth of human breast cancer cells. Cancer Res. 47:1566–70.
Acknowledgments
This study was supported by grants for the National High Technology Research and Development Program of China (2004AA2Z3170, 2005AA2Z3D40) (863 Program), the National Basic Research Program (2003CCA04300) (973 Program) and a project grant (03007) from the Department of Education of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Yao, Z., Che, Xc., Lu, R. et al. Inhibition by Tyroserleutide (YSL) on the Invasion and Adhesion of the Mouse Melanoma Cell. Mol Med 13, 14–21 (2007). https://doi.org/10.2119/2006-00061.Yao
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/2006-00061.Yao