Abstract
Background
Behcet’s disease (BD) is a chronic, relapsing, systemic vasculitis of unknown etiology. There is an increased predisposition to insulin resistance and metabolic syndrome (MetS) in BD patients.
Objective
The aim of this study was to determine serum salusin-α and salusin-β levels in BD patients and healthy controls and to investigate their association with MetS.
Patients and Methods
Twenty-five BD patients and 25 healthy controls were included in the study. Salusin-α and salusin-β levels were measured in blood samples using ELISA. In addition, BD patients and healthy controls were evaluated in terms of MetS.
Results
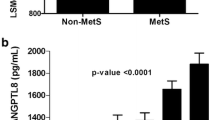
The mean serum salusin-α level in BD patients was significantly lower compared to healthy controls (p = 0.03), whereas the mean serum salusin-β level in BD patients was significantly higher compared to healthy controls (p = 0.03). The mean serum salusin-α level was significantly lower in BD patients with MetS compared to BD patients without MetS (p = 0.04).
Conclusions
Serum salusin-α level (an anti-atherogenic molecule) was lower, while serum salusin-β level (a pro-atherogenic molecule) was higher in BD patients. We consider that the decrease in salusin-α and the increase in salusin-β levels contribute to the development of MetS.
Similar content being viewed by others
References
Evereklioglu C. Current concepts in the etiology and treatment of Behcet disease. Surv Ophthalmol 2005; 50: 297–350.
Alpsoy E, Zouboulis CC, Ehrlich GE. Mucocutaneous lesions of Behcet’s disease. Yonsei Med J 2007; 48: 573–85.
Erdem H, Dinc A, Pay S, Simsek I, Turan M. Peripheral insulin resistance in patients with Behcet’s disease. J Eur Acad Dermatol Venereol 2006; 20: 391–5.
Kim SK, Choe JY, Park SH, Lee SW, Lee GH, Chung WT. Increased insulin resistance and serum resistin in Korean patients with Behcet’s disease. Arch Med Res 2010; 41: 457.
Gül A. Behcet’s disease: an update on the pathogenesis. Clin Exp Rheumatol 2001; 19: 6–12.
Sandkamp M, Funke H, Schulte H, Konler E, Assmann G. Lipoprotein (a) is an independent risk factor for myocardial infarction at a young age. Clin Chem 1990; 36: 20–3.
Shichiri M, Ishimaru S, Ota T, Nishikawa T, Isogai T, Hirata Y. Salusins: newly identified bioactive peptides with hemodynamic and mitogenic activities. Nat Med 2003; 9: 1166–72.
Tatemoto K, Hosoya M, Habata Y, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun 1998; 20: 471–6.
Kimoto S, Sato K, Watanabe T, Suguro T, Koyama T, Shichiri M. Serum levels and urinary excretion of salusin-alpha in renal insufficiency. Regul Pept 2010; 8: 129–32.
Sato K, Fujimoto K, Koyama T, Shichiri M. Release of salusin-beta from human monocytes/macrophages. Regul Pept 2010; 162: 68–72.
Nakayama C, Shichiri M, Sato K, Hirata Y. Expression of prosalusin in human neuroblastoma cells. Peptides 2009; 30: 1362–7.
International Study Group for Behcet’s Disease: Criteria for diagnosis of Behcet’s disease. Lancet 1990; 335: 1078–80.
Yazici C, Köse K, Calis¸ M, DemIr M, Kirnap M, Ates F. Increased advanced oxidation protein products in Behcet’s disease: a new activity marker? Br J Dermatol 2004; 151: 105–11.
Sidiropoulos PI, Karvounaris SA, Boumpas DT. Metabolic syndrome in rheumatic diseases: epidemiology, pathophysiology, and clinical implications. Arthritis Res Ther 2008; 10: 207.
Kondo N, Nomura M, Nakaya Y, Ito S, Ohguro T. Association of Inflammatory marker and highly sensitive c reactive protein with aerobic exercise capacity, maximum oxygen uptake and insulin resistance in healthy middle aged volunteers. Circ J 2005; 69: 452–7.
Stengel A, Keire D, Goebel M, et al. The RAPID method for blood processing yields new insight in plasma concentrations and molecular forms of circulating gut peptides. Endocrinology 2009; 150: 5113–8.
Nakayama C, Shichiri M, Sato K, Hirata Y. Expression of prosalusin in human neuroblastoma cells. Peptides 2009; 30: 1362–7.
Watanabe T, Suguro T, Sato K, et al. Serum salusin-alpha levels are decreased and correlated negatively with carotid atherosclerosis in essential hypertensive patients. Hypertens Res 2008; 31: 463–8.
Watanabe T, Nishio K, Kanome T, et al. Impact of salusin alpha and beta on human macrophage foam cell formation and coronary atherosclerosis. Circulation 2008; 117: 638–48.
Watanabe T, Sato K, Itoh F, et al. The roles of salusins in atherosclerosis and related cardiovascular diseases. J Am Soc Hypertens 2011; 5: 359–65.
Ti Y, Wang F, Wang ZH, et al. Associations of serum salusin-alpha levels with atherosclerosis and left ventricular diastolic dysfunction in essential hypertension. J Hum Hypertens 2012; 26: 603–9.
Geri G, Wechsler B, Thi Huongdu L, et al. Spectrum of cardiac lesions in Behcet disease: a series of 52 patients and review of the literature. Medicine (Baltimore) 2012; 91: 25–34.
Ozgen M, Koca SS, Dagli N, Balin M, Ustundag B, Isik A. Serum salusin-alpha level in rheumatoid arthritis. Regulatory Peptides 2011; 167: 125–8.
Aydin S, Celik O, Gurates B, et al. Concentrations of preptin, salusins and hepcidins in plasma and milk of lactating women with or without gestational diabetes mellitus. Peptides 2013; 49: 123–30.
Celik E, Celik O, Yilmaz E, et al. Association of low maternal levels of salusins with gestational diabetes mellitus and with small-for-gestational-age fetuses. Eur J Obstet Gynecol Reprod Biol 2013; 167: 29–33.
Aydin S, Aydin S. Salusin-alpha and -beta expression in heart and aorta with and without metabolic syndrome. Biotech Histochem 2014; 89: 98–103.
Direskeneli H. Behcet’s disease: infectious aetiology, new autoantigens, and HLA-B51. Ann Rheum Dis 2001; 60: 996–1002.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Erden, I., Demir, B., Uçak, H. et al. Serum salusin-α and salusin-β levels in patients with Behcet’s disease. Eur J Dermatol 24, 577–582 (2014). https://doi.org/10.1684/ejd.2014.2397
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1684/ejd.2014.2397