Abstract
Ischemic stroke presents a leading cause of mortality and morbidity worldwide. Theaflavic acid (TFA) is a theaflavin isolated from black tea that exerts a potentially neuro-protective effect. However, the dynamic properties of TFA-mediated protection remain largely unknown. In the current study, we evaluated the function of TFA in the mitochondria apoptotic pathway using mathematical modeling. We found that TFA-enhanced B-cell lymphoma 2 (Bcl-2) overexpression can theoretically give rise to bistability. The bistability is highly robust against parametric stochasticity while also conferring considerable variability in survival threshold. Stochastic simulations faithfully match the TFA dose response pattern seen in experimental studies. In addition, we identified a dose- and time-dependent synergy between TFA and nimodipine, a clinically used neuro-protective drug. This synergistic effect was enhanced by bistability independent of temporal factors. Precise application of pulsed doses of TFA can also promote survival compared with sustained TFA treatment. These data collectively demonstrate that TFA treatment can give rise to bistability and that synergy between TFA and nimodipine may offer a promising strategy for developing therapeutic neuro-protection against ischemic stroke.
概要
目的
利用数学建模方法来研究茶黄素酸和尼莫地平在线粒体凋亡途径中的协同神经保护作用.
创新点
茶黄素酸诱导的 Bcl-2 表达在理论上可以引起系统性的双稳态. 此双稳态对模型参数变化具有很强的鲁棒性, 但同时又对生存状态阈值的变化具有相当的敏感性. 通过随机模拟, 我们可以很好地拟合实验中测定的茶黄素酸剂量反应. 此外, 我们确定了茶黄素酸和尼莫地平 (一种临床应用的神经保护药物) 之间的剂量和时间依赖性协同作用, 这种协同效应通过双稳态得到增强.
方法
采用微分方程描述分子间相互作用; 运用局部敏感性分析探讨系统参数敏感性; 运用 Bliss 和 Loewe 联合指数计算茶黄素酸和尼莫地平的协同性; 使用 MATCONT 工具箱揭示系统的分岔性质.
结论
与持续的茶黄素酸处理相比, 对细胞给予脉冲样茶黄素酸处理可以显著提高细胞生存率. 这些数据表明, 茶黄素酸可以诱导系统产生双稳态, 同时茶黄素酸与尼莫地平的协同作用可能为缺血性中风的治疗提供了一种有效的方式.
Similar content being viewed by others
References
Albeck JG, Burke JM, Spencer SL, et al., 2008. Modeling a snap-action, variable-delay switch controlling extrinsic cell death. PLoS Biol, 6(12):2831–2852. https://doi.org/10.1371/journal.pbio.0060299
Bagci EZ, Vodovotz Y, Billiar TR, et al., 2006. Bistability in apoptosis: roles of Bax, Bcl-2, and mitochondrial permeability transition pores. Biophys J, 90(5):1546–1559. https://doi.org/10.1529/biophysj.105.068122
Chen C, Cui J, Lu HZ, et al., 2007. Modeling of the role of a Bax-activation switch in the mitochondrial apoptosis decision. Biophys J, 92(12):4304–4315. https://doi.org/10.1529/biophysj.106.099606
Chong SJF, Low ICC, Pervaiz S, 2014. Mitochondrial ROS and involvement of Bcl-2 as a mitochondrial ROS regulator. Mitochondrion, 19:39–48. https://doi.org/10.1016/j.mito.2014.06.002
Chong SJF, Marchi S, Petroni G, et al., 2020. Noncanonical cell fate regulation by Bcl-2 proteins. Trends Cell Biol, 30(7):537–555. https://doi.org/10.1016/j.tcb.2020.03.004
Coling DE, Yu KCY, Somand D, et al., 2003. Effect of SOD1 overexpression on age- and noise-related hearing loss. Free Radic Biol Med, 34(7):873–880. https://doi.org/10.1016/s0891-5849(02)01439-9
Cui J, Chen C, Lu HZ, et al., 2008. Two independent positive feedbacks and bistability in the Bcl-2 apoptotic switch. PLoS ONE, 3(1):e1469. https://doi.org/10.1371/journal.pone.0001469
Dejean LM, Martinez-Caballero S, Guo L, et al., 2005. Oligomeric Bax is a component of the putative cytochrome c release channel MAC, mitochondrial apoptosis-induced channel. Mol Biol Cell, 16(5):2424–2432. https://doi.org/10.1091/mbc.E04-12-1111
Dhooge A, Govaerts W, Kuznetsov YA, 2003. MATCONT: a MATLAB package for numerical bifurcation analysis of ODEs. ACM Trans Math Softw, 29(2):141–164. https://doi.org/10.1145/779359.779362
Fitzgerald JB, Schoeberl B, Nielsen UB, et al., 2006. Systems biology and combination therapy in the quest for clinical efficacy. Nat Chem Biol, 2(9):458–466. https://doi.org/10.1038/nchembio817
Görlach A, Bertram K, Hudecova S, et al., 2015. Calcium and ROS: a mutual interplay. Redox Biol, 6:260–271. https://doi.org/10.1016/j.redox.2015.08.010
Ho KL, Harrington HA, 2010. Bistability in apoptosis by receptor clustering. PLoS Comput Biol, 6(10):e1000956. https://doi.org/10.1371/journal.pcbi.1000956
Jiang H, Zhang LJ, Kuo J, et al., 2005. Resveratrol-induced apoptotic death in human U251 glioma cells. Mol Cancer Ther, 4(4):554–561. https://doi.org/10.1158/1535-7163.MCT-04-0056
Keizer EM, Bastian B, Smith RW, et al., 2019. Extending the linear-noise approximation to biochemical systems influenced by intrinsic noise and slow lognormally distributed extrinsic noise. Phys Rev E, 99(5):052417. https://doi.org/10.1103/PhysRevE.99.052417
Koubi D, Jiang H, Zhang LJ, et al., 2005. Role of Bcl-2 family of proteins in mediating apoptotic death of PC12 cells exposed to oxygen and glucose deprivation. Neurochem Int, 46(1):73–81. https://doi.org/10.1016/j.neuint.2004.06.006
Legewie S, Blüthgen N, Herzel H, 2006. Mathematical modeling identifies inhibitors of apoptosis as mediators of positive feedback and bistability. PLoS Comput Biol, 2(9):e120. https://doi.org/10.1371/journal.pcbi.0020120
Legrand AJ, Konstantinou M, Goode EF, et al., 2019. The diversification of cell death and immunity: Memento mori. Mol Cell, 76(2):232–242. https://doi.org/10.1016/j.molcel.2019.09.006
Letai A, Bassik MC, Walensky LD, et al., 2002. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell, 2(3):183–192. https://doi.org/10.1016/S1535-6108(02)00127-7
Li LX, Yiin GS, Geraghty OC, et al., 2015. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurol, 14(9):903–913. https://doi.org/10.1016/S1474-4422(15)00132-5
Li QJ, Ji ZL, Li K, 2018. Effect of combination of parenteral edaravone and nimodipine on ischemic cerebral injury following cerebral hemorrhage. Trop J Pharm Res, 17(5):955–960. https://doi.org/10.4314/tjpr.v17i5.27
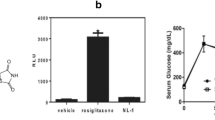
Li Y, Shi J, Sun XT, et al., 2020. Theaflavic acid from black tea protects PC12 cells against ROS-mediated mitochondrial apoptosis induced by OGD/R via activating Nrf2/ARE signaling pathway. J Nat Med, 74:238–246. https://doi.org/10.1007/s11418-019-01333-4
Liu CQ, Zhou RX, Sun SG, 2004. Nimodipine modulates Bcl-2 and Bax mRNA expression after cerebral ischemia. J Huazhong Univ Sci Technol Med Sci, 24(2):170–172. https://doi.org/10.1007/BF02885421
Liu L, Huang WW, Wang JH, et al., 2017. Anthraquinone derivative exerted hormetic effect on the apoptosis in oxygen-glucose deprivation-induced PC12 cells via ERK and Akt activated Nrf2/HO-1 signaling pathway. Chem Biol Interact, 262:1–11. https://doi.org/10.1016/j.cbi.2016.12.001
Luo YG, Yang XF, Zhao ST, et al., 2013. Hydrogen sulfide prevents OGD/R-induced apoptosis via improving mitochondrial dysfunction and suppressing an ROS-mediated caspase-3 pathway in cortical neurons. Neurochem Int, 63(8):826–831. https://doi.org/10.1016/j.neuint.2013.06.004
Mehta SL, Manhas N, Raghubir R, 2007. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev, 54(1):34–66. https://doi.org/10.1016/j.brainresrev.2006.11.003
Niture SK, Jaiswal AK, 2012. Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J Biol Chem, 287(13):9873–9886. https://doi.org/10.1074/jbc.M111.312694
Pellegrini-Giampietro DE, Mannaioni G, Bagetta G, 2009. Post-ischemic brain damage: the endocannabinoid system in the mechanisms of neuronal death. FEBS J, 276(1):2–12. https://doi.org/10.1111/j.1742-4658.2008.06765.x
Roine RO, Kaste M, Kinnunen A, et al., 1990. Nimodipine after resuscitation from out-of-hospital ventricular fibrillation: a placebo-controlled, double-blind, randomized trial. JAMA, 264(24):3171–3177. https://doi.org/10.1001/jama.1990.03450240073043
Roux J, Hafner M, Bandara S, et al., 2015. Fractional killing arises from cell-to-cell variability in overcoming a caspase activity threshold. Mol Syst Biol, 11(5):803. https://doi.org/10.15252/msb.20145584
Saito A, Maier CM, Narasimhan P, et al., 2005. Oxidative stress and neuronal death/survival signaling in cerebral ischemia. Mol Neurobiol, 31(1–3): 105–116. https://doi.org/10.1385/MN:31:1-3:105
Seidman MD, 2000. Effects of dietary restriction and antioxidants on presbyacusis. Laryngoscope, 110(5):727–738. https://doi.org/10.1097/00005537-200005000-00003
Seidman MD, Khan MJ, Bai U, et al., 2000. Biologic activity of mitochondrial metabolites on aging and age-related hearing loss. Am J Otol, 21(2):161–167. https://doi.org/10.1016/s0196-0709(00)80003-4
Seidman MD, Khan MJ, Tang WX, et al., 2002. Influence of lecithin on mitochondrial DNA and age-related hearing loss. Otolaryngol Head Neck Surg, 127(3):138–144. https://doi.org/10.1067/mhn.2002.127627
Seidman MD, Ahmad N, Joshi D, et al., 2004. Age-related hearing loss and its association with reactive oxygen species and mitochondrial DNA damage. Acta Otolaryngol Suppl, (552):16–24. https://doi.org/10.1080/03655230410017823
Spencer SL, Sorger PK, 2011. Measuring and modeling apoptosis in single cells. Cell, 144(6):926–939. https://doi.org/10.1016/j.cell.2011.03.002
Straetemans R, O’Brien T, Wouters L, et al., 2005. Design and analysis of drug combination experiments. Biom J, 47(3):299–308. https://doi.org/10.1002/bimj.200410124
Sun TZ, Chen C, Wu YY, et al., 2009. Modeling the role of p53 pulses in DNA damage-induced cell death decision. BMC Bioinformatics, 10:190. https://doi.org/10.1186/1471-2105-10-190
Sun TZ, Lin XZ, Wei YN, et al., 2010. Evaluating bistability of Bax activation switch. FEBS Lett, 584(5):954–960. https://doi.org/10.1016/j.febslet.2010.01.034
Thompson JW, Narayanan SV, Perez-Pinzon MA, 2012. Redox signaling pathways involved in neuronal ischemic preconditioning. Curr Neuropharmacol, 10(4):354–369. https://doi.org/10.2174/157015912804143577
Tian TH, Burrage K, 2006. Stochastic models for regulatory networks of the genetic toggle switch. Proc Natl Acad Sci USA, 103(22):8372–8377. https://doi.org/10.1073/pnas.0507818103
Tsujimoto Y, 2003. Cell death regulation by the Bcl-2 protein family in the mitochondria. J Cell Physiol, 195(2):158–167. https://doi.org/10.1002/jcp.10254
Tyson JJ, Chen KC, Novak B, 2003. Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr Opin Cell Biol, 15(2):221–231. https://doi.org/10.1016/S0955-0674(03)00017-6
Willis SN, Adams JM, 2005. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol, 17(6):617–625. https://doi.org/10.1016/j.ceb.2005.10.001
Yang CJ, Hawkins KE, Doré S, et al., 2019. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol, 316(2):C135–C153. https://doi.org/10.1152/ajpcell.00136.2018
Yin ZY, Qi H, Liu LL, et al., 2017. The optimal regulation mode of Bcl-2 apoptotic switch revealed by bistability analysis. Biosystems, 162:44–52. https://doi.org/10.1016/j.biosystems.2017.09.011
Youle RJ, Strasser A, 2008. The BCL-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol, 9(1):47–59. https://doi.org/10.1038/nrm2308
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Nos. 31971185 and 31800316), the Key Projects of Support Program for Outstanding Young Talents in Colleges and Universities of Anhui Province (Nos. gxyqZD2020031 and gxyq2018034), and the Key Project of the Education Department of Anhui Province, China (No. KJ2017A359).
We thank Dr. Liangliang ZHU (School of Life Sciences, the Province Key Laboratory of the Biodiversity Study and Ecology Conservation in Southwest Anhui, Anqing Normal University, Anhui, China) for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
Dan MU and Tingzhe SUN conceptualized the study, performed project administration, and acquired the funding; Dan MU, Huaguang QIN, and Tingzhe SUN developed the methodology and wrote the manuscript; Dan MU, Huaguang QIN, Mengjie JIAO, Shaogui HUA, and Tingzhe SUN validated the study, performed the data analysis and curation, and reviewed the draft; Tingzhe SUN supervised the study. All authors have read and agreed to the published version of the manuscript, and therefore, have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Dan MU, Huaguang QIN, Mengjie JIAO, Shaogui HUA, and Tingzhe SUN declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
Supplementary materials
Rights and permissions
About this article
Cite this article
Mu, D., Qin, H., Jiao, M. et al. Modeling the neuro-protection of theaflavic acid from black tea and its synergy with nimodipine via mitochondria apoptotic pathway. J. Zhejiang Univ. Sci. B 22, 123–135 (2021). https://doi.org/10.1631/jzus.B2000540
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B2000540