Abstract
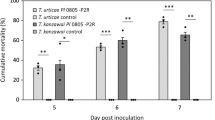
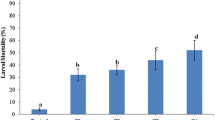
Xenorhabdus bacterium has been used as a biological control agent against Luciaphorus sp., a mushroom mite endemic in Thailand. To develop an effective formulation of Xenorhabdus stokiae, treatments using different parts of X. stokiae isolate PB09 culture, including whole cell suspension, cell-free supernatant, and crude cell extract, were performed. The results show that different parts of X. stokiae isolate PB09 culture could induce variable effects on mite mortality and fecundity. Application with cell-free supernatant of X. stokiae culture resulted in both the highest mite mortality rate [(89.00±3.60)%] and the lowest mite fecundity [(41.33±23.69) eggs/gravid female]. Whole cell suspension of X. stokiae isolate PB09 culture was found to be slightly less effective than its cell-free supernatant, suggesting that X. stokiae was more likely to release its metabolites with acaricidal activities to the surrounding culture media. Crude cell extract of X. stokiae was not effective against mites. Cell-free supernatant of X. stokiae isolate PB09 was the most effective biological control agent and it could be conveniently used in future formulations instead of live bacteria.
Similar content being viewed by others
References
Bjørnson, S., Keddie, B.A., 2001. Disease prevalence and transmission of Microsporidium phytoseiuli infecting the predatory mite, Phytoseiulus persimilis (Acari: Phytoseiidae). J. Invertebr. Pathol., 77(2):114–119. [doi:10.1006/jipa.2001.5008]
Bode, H.B., 2009. Entomopathogenic bacteria as a source of secondary metabolites. Curr. Opin. Chem. Biol., 13(2): 224–230. [doi:10.1016/j.cbpa.2009.02.037]
Bowen, D., Blackburn, M., Rocheleau, T., Grutzmacher, C., Ffrench-Constant, R.H., 2000. Secreted proteases from Photorhabdus luminescens: separation of the extracellular proteases from the insecticidal Tc toxin complexes. Insect Biochem. Mol. Biol., 30(1):69–74. [doi:10.1016/S0965-1748(99)00098-3]
Brivio, M.F., Mastore, M., Nappi, A.J., 2010. A pathogenic parasite interferes with phagocytosis of insect immunocompetent cells. Dev. Comp. Immunol., 34(9):991–998. [doi:10.1016/j.dci.2010.05.002]
Bro-Rasmussen, F., 1996. Contamination by persistent chemicals in food chain and human health. Sci. Total Environ., 188:S45–S60. [doi:10.1016/0048-9697(96)05 276-X]
Bussaman, P., Chandrapatya, A., Sermswan, R.W., Grewal, P.S., 2004. Morphology, Biology and Behavior of the Genus Pygmephorus (Acari: Heterostigmata), a New Parasite of Economic Edible Mushroom. Proceeding of XXII International Congress of Entomology. Carillon Conference Management Pty Ltd., 15–21 August, Brisbane, Australia.
Bussaman, P., Sermswan, R.W., Grewal, P.S., 2006. Toxicity of the entomopathogenic bacteria Photorhabdus and Xenorhabdus to the mushroom mite (Luciaphorus sp.; Acari: Pygmephoridae). Biocontrol Sci. Technol., 16(3): 245–256. [doi:10.1080/09583150500335822]
Bussaman, P., Sobanboa, S., Grewal, P.S., Chandrapatya, A., 2009. Pathogenicity of additional strains of Photorhabdus and Xenorhabdus (Enterobacteriaceae) to the mushroom mite Luciaphorus perniciosus (Acari: Pygmephoridae). Appl. Entomol. Zool., 44(2):293–299. [doi:10.1303/aez.2009.293]
Caldas, C., Pereira, A., Cherqui, A., Simoes, N., 2002. Purification and characterization of an extracellular protease from Xenorhabdus nematophila involved in insect immunosuppression. Appl. Environ. Microb., 68(3):1297–1304. [doi:10.1128/AEM.68.3.1297-1304.2002]
Campos-Herrera, R., Tailliez, P., Pages, S., Ginibre, N., Gutierrez, C., Boemare, N.E., 2009. Characterization of Xenorhabdus isolates from La Rioja (Northern Spain) and virulence with and without their symbiotic entomopathogenic nematodes (Nematoda: Steinernematidae). J. Invertebr. Pathol., 102(2):173–181. [doi:10.1016/j.jip.2009.08.007]
Cho, S., Kim, Y.H., 2004. Hemocyte apoptosis induced by entomopathogenic bacteria, Xenorhabdus and Photorhabdus, in Bombyx mori. J. Asia-Pacific Entomol., 7(2):195–200. [doi:10.1016/S1226-8615(08)60215-0]
Chongchitmate, P., Somsook, V., Hormchan, P., Visarathanonth, N., 2005. Bionomics of entomopathogenic nematode Steinernema siamkayai Stock, Somsook and Reid (n. sp.) and its efficacy against Helicoverpa armigera Hübner (Lepidoptera: Noctuidae). Kasetsart J. (Nat. Sci.), 39(3): 431–439.
Gaugler, R., 2002. Entomopathogenic Nematology. CABI Publishing, CAB International, Wallingford, Oxfordshire, UK. [doi:10.1079/9780851995670.0000]
Grewal, P.S., Ehlers, R.U., Shapiro-Ilan, D.I., 2005. Nematodes as Biocontrol Agents. CABI Publishing, CAB International, Wallingford, Oxfordshire, UK. [doi:10.1079/9780851990170.0000]
Ji, D., Yi, Y., Kang, G.H., Choi, Y.H., Kim, P., Baek, N.I., Kim, Y., 2004. Identification of an antibacterial compound, benzylideneacetone, from Xenorhabdus nematophila against major plant-pathogenic bacteria. FEMS Microbiol. Lett., 239(2):241–248. [doi:10.1016/j.femsle.2004.08.041]
Kaya, H.K., Stock, S.P., 1997. Techniques in Insect Nematology. In: Lacey, L.A. (Ed.), Manual of Techniques in Insect Pathology. Academic Press, London, UK, p.281–324.
Klement, Z., Rudolph, K., Sands, D.C. (Eds.), 1990. Method in Phytobacteriology. Academiai Kiado, Budapest, Hungary, p.99–100.
Lacey, L.A., 1997. Manual of Techniques in Insect Pathology: Biological Techniques Series. Academic Press, San Diego, California, USA, p.315–322.
Mahar, A.N., Munir, M., Mahar, A.Q., 2004. Studies of different application methods of Xenorhabdus and Photorhabdus cells and their toxin in broth solution to control locust (Schistocerca gregaria). Asian J. Plant Sci., 3(6):690–695. [doi:10.3923/ajps.2004.690.695]
Mahar, A.N., Munir, M., Elawad, S., Gowen, S.R., Hague, N.G.M., 2005. Pathogenicity of bacterium, Xenorhabdus nematophila isolated from entomopathogenic nematode (Steinernema carpocapsae) and its secretion against Galleria mellonella larvae. J. Zhejiang Univ.-Sci. B, 6(6): 457–463. [doi:10.1631/jzus.2005.B0457]
Mahar, A.N., Jan, N.D., Mahar, G.M., Mahar, A.Q., 2008. Control of insects with entomopathogenic bacterium Xenorhabdus nematophila and its toxic secretions. Int. J. Agric. Biol., 10(1):52–56.
Mohamed, M.A., 2007. Purification and characterization of an alkaline protease produced by the bacterium Xenorhabdus nematophila BA2, a symbiont of entomopathogenic nematode Steinernema carpocapsae. Res. J. Agric. Biol. Sci., 3(5):510–521.
Morgan, J.A., Sergeant, M., Ellis, D., Ousley, M., Jarrett, P., 2001. Sequence analysis of insecticidal gene from Xenorhabdus nematophila PME1296. Appl. Environ. Microbiol., 67(5):2062–2069. [doi:10.1128/AEM.67.5. 2062-2069.2001]
Sharma, S., Waterfield, N., Bowen, D., Rocheleau, T., Holland, L., James R., Ffrench-Constant, R., 2002. The lumicins: novel bacteriocins from Photorhabdus luminescens with similarity to the uropathogenic-specific protein (USP) from uropathogenic Escherichia coli. FEMS Microbiol. Lett., 214(2):241–249. [doi:10.1111/j.1574-6968.2002. tb11354.x]
Shrestha, S., Kim, Y., 2010. Differential pathogenicity of two entomopathogenic bacteria, Photorhabdus temperata subsp. temperata and Xenorhabdus nematophila against the red flour beetle, Tribolium castaneum. J. Asian-Pacific Entomol., 13(3):209–213. [doi:10.1016/j.aspen.2010.04.002]
Stock, S.P., Somsook, V., Reid, A.P., 1998. Steinernema siamkayai n. sp. (Rhabditida: Steinernematidae), an entomopathogenic nematode from Thailand. Syst. Parasitol., 41(2):105–113. [doi:10.1023/A:1006087017195]
Vala, F., Egas, M., Sabelis, M.W., 2004. Wolbachia affects oviposition and mating behavior of its spider mite host. J. Evol. Biol., 17(3):692–700. [doi:10.1046/j.1420-9101.2003.00679.x]
Author information
Authors and Affiliations
Corresponding author
Additional information
Project (No. RTA 4880006) supported by the Thailand Research Fund, Kasetsart University and Mahasarakham University
Rights and permissions
About this article
Cite this article
Bussaman, P., Sa-Uth, C., Rattanasena, P. et al. Acaricidal activities of whole cell suspension, cell-free supernatant, and crude cell extract of Xenorhabdus stokiae against mushroom mite (Luciaphorus sp.). J. Zhejiang Univ. Sci. B 13, 261–266 (2012). https://doi.org/10.1631/jzus.B1100155
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1100155