Abstract
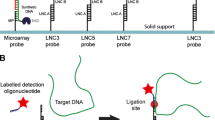
The aim of this study was to develop and validate an oligonucleotide suspension array for rapid identification of 15 bacterial species responsible for bacteremia, particularly prevalent in Chinese hospitals. The multiplexed array, based on the QIAGEN LiquiChip Workstation, included 15 oligonucleotide probes which were covalently bound to different bead sets. PCR amplicons of a variable region of the bacterial 23S rRNA genes were hybridized to the bead-bound probes. Thirty-eight strains belonging to 15 species were correctly identified on the basis of their corresponding species-specific hybridization profiles. The results show that the suspension array, in a single assay, can differentiate isolates over a wide range of strains and species, and suggest the potential utility of suspension array system to clinical laboratory diagnosis.
Similar content being viewed by others
References
Anthony, R.M., Brown, T.J., French, G.L., 2000. Rapid diagnosis of bacteremia by universal amplification of 23S ribosomal DNA followed by hybridization to an oligonucleotide array. J. Clin. Microbiol., 38(2):781–788.
Armstrong, B., Stewart, M., Mazumder, A., 2000. Suspension arrays for high throughput, multiplexed single nucleotide polymorphism genotyping. Cytometry, 40(2):102–108. [doi:10.1002/(SICI)1097-0320(20000601)40:2<102::AID-C YTO3>3.3.CO;2-W]
Bovers, M., Diaz, M.R., Hagen, F., Spanjaard, L., Duim, B., Visser, C.E., Hoogveld, H.L., Scharringa, J., Hoepelman, I.M., Fell, J.W., Boekhout, T., 2007. Identification of genotypically diverse Cryptococcus neoformans and Cryptococcus gattii isolates by Luminex xMAP technology. J. Clin. Microbiol., 45(6):1874–1883. [doi:10.1128/JCM.00223-07]
Bryant, P.A., Venter, D., Robins-Browne, R., Curtis, N., 2004. Chips with everything: DNA microarrays in infectious diseases. Lancet Infect. Dis., 4(2):100–111. [doi:10.1016/S1473-3099(04)00930-2]
Cai, H., White, P.S., Torney, D., Deshpande, A., Wang, Z., Keller, R.A., Marrone, B., Nolan, J.P., 2000. Flow cytometry-based minisequencing: a new platform for high-throughput single-nucleotide polymorphism scoring. Genomics, 66(2):135–143. [doi:10.1006/geno.2000.6218]
Christensen, H., Nordentoft, S., Olsen, J., 1998. Phylogenetic relationships of Salmonella based on rRNA sequences. Int. J. Syst. Bacteriol., 48(Pt 2):605–610.
Corless, C.E., Guiver, M., Borrow, R., Edwards-Jones, V., Kaczmarski, E.B., Fox, A.J., 2000. Contamination and sensitivity issues with a real-time universal 16S rRNA PCR. J. Clin. Microbiol., 38(5):1747–1752.
Dauga, C., 2002. Evolution of the gyrB gene and the molecular phylogeny of Enterobacteriaceae: a model molecule for molecular systematic studies. Int. J. Syst. Evol. Microbiol., 52(Pt 2):531–547.
Ding, L.P., Sun, Y.H., Wang, Q., Nian, H., 2004. Drug resistance of common bacteria isolated from blood and bone marrow. J. Chin. Med. Univ., 33(1):83–85 (in Chinese).
Dunbar, S., Godbout, R., Newkirk, H., Hetzel, J., 2003a. Microsphere suspension array technology for SNP detection in cattle. IEEE Eng. Med. Biol. Mag., 22(4):158–162. [doi:10.1109/MEMB.2003.1237526]
Dunbar, S.A., Vander Zee, C.A., Oliver, K.G., Karem, K.L., Jacobson, J.W., 2003b. Quantitative, multiplexed detection of bacterial pathogens: DNA and protein applications of the Luminex LabMAP system. J. Microbiol. Methods, 53(2):245–252. [doi:10.1016/S0167-7012(03)00028-9]
Fukushima, M., Kakinuma, K., Hayashi, H., Nagai, H., Ito, K., Kawaguchi, R., 2003. Detection and identification of Mycobacterium species isolates by DNA microarray. J. Clin. Microbiol., 41(6):2605–2615. [doi:10.1128/JCM.41.6.2605-2615.2003]
Henry, M.R., Wilkins Stevens, P., Sun, J., Kelso, D.M., 1999. Real-time measurements of DNA hybridization on microparticles with fluorescence resonance energy transfer. Anal. Biochem., 276(2):204–214. [doi:10.1006/abio.1999.4344]
Kettman, J.R., Davies, T., Chandler, D., Oliver, K.G., Fulton, R.J., 1998. Classification and properties of 64 multiplexed microsphere sets. Cytometry, 33(2):234–243. [doi:10.1002/(SICI)1097-0320(19981001)33:2<234::AID-C YTO19>3.0.CO;2-V]
Khayr, W.F., CarMichael, M.J., Dubanowich, C.S., Latif, R.H., 2003. Epidemiology of bacteremia in the geriatric population. Am. J. Ther., 10(2):127–131. [doi:10.1097/00045391-200303000-00008]
Kwok, S., Higuchi, R., 1989. Avoiding false positives with PCR. Nature, 339(6221):237–238. [doi:10.1038/339237a0]
Leffers, H., Kjems, J., Ostergaard, L., Larsen, N., Garrett, R.A., 1987. Evolutionary relationships amongst archaebacteria. A comparative study of 23S ribosomal RNAs of a sulphur-dependent extreme thermophile, an extreme halophile and a thermophilic methanogen. J. Mol. Biol., 195(1):43–61. [doi:10.1016/0022-2836(87)90326-3]
Maiwald, M., Ditton, H.J., Sonntag, H.G., von Knebel Doeberitz, M., 1994. Characterization of contaminating DNA in Taq polymerase which occurs during amplification with a primer set for Legionella 5S ribosomal RNA. Mol. Cell. Probes, 8(1):11–14. [doi:10.1006/mcpr.1994.1002]
Nolan, J.P., Mandy, F.F., 2001. Suspension array technology: new tools for gene and protein analysis. Cell. Mol. Biol. (Noisy-le-grand), 47(7):1241–1256.
Nolan, J.P., Sklar, L.A., 2002. Suspension array technology: evolution of the flat-array paradigm. Trends Biotechnol., 20(1):9–12. [doi:10.1016/S0167-7799(01)01844-3]
Peters, R.P., van Agtmael, M.A., Danner, S.A., Savelkoul, P.H., Vandenbroucke-Grauls, C.M., 2004. New developments in the diagnosis of bloodstream infections. Lancet Infect. Dis., 4(12):751–760. [doi:10.1016/S1473-3099(04)01205-8]
Schmitt, M., Bravo, I.G., Snijders, P.J., Gissmann, L., Pawlita M., Waterboer, T., 2006. Bead-based multiplex genotyping of human papillomaviruses. J. Clin. Microbiol., 44(2):504–512. [doi:10.1128/JCM.44.2.504-512.2006]
Troesch, A., Nguyen, H., Miyada, C.G., Desvarenne, S., Gingeras, T.R., Kaplan, P.M., Cros, P., Mabilat, C., 1999. Mycobacterium species identification and rifampin resistance testing with high-density DNA probe arrays. J. Clin. Microbiol., 37(1):49–55.
Vandamme, P., Pot, B., Gillis, M., de Vos, P., Kersters, K., Swings, J., 1996. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol. Rev., 60(2):407–438.
Wetmur, J.G., 1991. DNA probes: applications of the principles of nucleic acid hybridization. Crit. Rev. Biochem. Mol. Biol., 26(3):227–259. [doi:10.3109/10409239109114069]
Woese, C.R., 1987. Bacterial evolution. Microbiol. Rev., 51(2):221–271.
Zehr, J.P., Crumbliss, L.L., Church, M.J., Omoregie, E.O., Jenkins, B.D., 2003. Nitrogenase genes in PCR and RT-PCR reagents: implications for studies of diversity of functional genes. Biotechniques, 35(5):996–1002, 1004–1005.
Author information
Authors and Affiliations
Corresponding author
Additional information
The two authors contributed equally to this work
Project (Nos. 2003C13015 and 021103128) supported by Science and Technology Department of Zhejiang Province, China
Rights and permissions
About this article
Cite this article
Hou, Xl., Jiang, Hl., Cao, Qy. et al. Using oligonucleotide suspension arrays for laboratory identification of bacteria responsible for bacteremia. J. Zhejiang Univ. Sci. B 9, 291–298 (2008). https://doi.org/10.1631/jzus.B0710470
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B0710470