Abstract
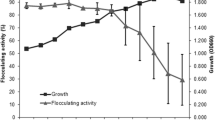
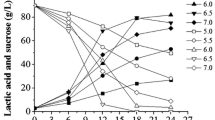
The production of elastase by Bacillus sp. EL31410 at various temperatures was investigated. In order to study the effect of temperature on elastase fermentation, different cultivation temperatures, ranging from 39 °C to 28 °C, were evaluated in shake flask. The result indicated that 37 °C was best for cell growth at earlier stage; while maximum elastase activity was obtained when the cells were cultivated at 30 °C. This result was verified by batch fermentation in 5-L bioreactor under 37 °C and 30 °C temperature, respectively. The specific cell growth rate at 37 °C was higher than that at 30 °C during earlier stage of cultivation. The maximum value [5.5 U/(h·g DCW)] of elastase formation rate occurred at 24 h at 30 °C compared to 4.6 U/(h·g DCW) at 30 h at 37 °C. Based on these results, two-stage temperature shift strategy and oscillatory temperature cultivation mode were evaluated in the next study. When compared to single temperature of 37 °C or 30 °C, both two-stage temperature shift strategy and oscillatory temperature strategy improved biomass but did not yield the same result as expected for elastase production. The maximum biomass (both 8.6 g/L) was achieved at 30 h at 37 °C, but at 42 h using two-stage temperature cultivation strategy. The highest elastase production (652 U/ml) was observed at 30 °C in batch process. It was concluded that cultivation at constant temperature of 30 °C was appropriate for elastase production by Bacillus sp. EL31410.
Similar content being viewed by others
References
Beg, Q.K., Saxena, R.K., Gupta, R., 2002. De-repression and subsequent induction of protease synthesis by Bacillus mojavensis under fed-batch operations. Process Biochemistry, 37:1103–1109.
Chen, Q.H., He, G.Q., Molhtar, A.M.A., 2002. Optimization of medium composition for the production of elastase by Bacillus sp. EL31410 with response surface methodology. Enzyme Microbial Technol, 30:667–672.
Chen, Q.H., He, G.Q., Schwarz, P., 2004. Studies on cultivation kinetics for elastase production by Bacillus sp. EL31410. J Agric Food Chem, 52:3356–3359.
Chu, J., Li, Y.R., 2002. Regulation Technology of Modern Fermentation Industry. Chemistry Industry Press, Beijing, China, p.262–263 (in Chinese).
Feng, Y.Y., He, Z.M., Say, L.O., Hu, J.Y., Zhang, Z.G., Win, J.N., 2003. Optimization of agitation, aeration, and temperature conditions for maximum β-mannanase production. Enzyme Microbial Technol, 32:282–289.
Ke, N., Xiao, C.S., 2002. Research process of elastase produced by microorganism. Microbiology, 4:91–94 (in Chinese).
Mei, L.H., Yao, S.J., Lin, D.Q., 1999. Biochemistry Process Technology. Science Press, Beijing, China, p.185–186 (in Chinese).
Morihara, K., 1967. Elastolytic properties of various proteases from microbial origin. Arch Biochem Biophys, 120:68–78.
Sachar, L.A., 1955. Photometry method for estimation of elastase activity. Proc Soc Expeti Biol Med, 90:323–325.
Shibata, Y., Fujimura, S., Nakamura, T., 1993. Purification and partial characterization of an elastolytic serine protease of Prevotella intermedia. Appl Environm Microbial, 59:2107–2111.
Tsai, Y.C., Jung, R.Y., Lin, S.F., 1988. Production and further characterization of an alkaline elastase production by alkalophilic Bacillus strain YaB. Appl Environm Microbial, 1:3156–3161.
Zhang, L.X., Zhang, Y.F., Li, L.Y., 1997. Biochemistry Experiment and Technology. Advanced Education Press, Beijing, China, p.1–3 (in Chinese).
Zhang, J.A., Wei, X.S., Xie, D.M., Sun, Y., Liu, D.H., 2002. Effects of oscillatory temperature and sparging nitrogen gas or carbon dioxide during later stage on glycerol fermentation. J Chemical Industry and Engineering, 53:980–983.
Zheng, M.Y., Du, G.C., Guo, W.G., Chen, J., 2001. A temperature-shift strategy in batch microbial transglutaminase fermentation. Process Biochemistry, 36:525–530.
Zins, M.M., Zimprich, A.C., Petermann, R.S., Rust, L., 2001. Expression and partial characterization of an elastase from Chromobacterium Violaceum. Veterinary Microbiology, 80:63–74.
Author information
Authors and Affiliations
Corresponding author
Additional information
Project (No. 20276064) supported by the National Natural Science Foundation of China
Rights and permissions
About this article
Cite this article
He, Gq., Xu, Y., Chen, Qh. et al. Effect of temperature on batch elastase production by Bacillus sp. EL31410. J. Zheijang Univ.-Sci. 5, 1583–1589 (2004). https://doi.org/10.1631/jzus.2004.1583
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.2004.1583