Abstract
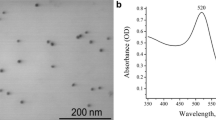
We have conjugated chloroquine onto nano-sized, thiol-stabilized gold nanoparticles by using 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC) / N-hydroxysulfosuccinimide (NHS) chemistry. The formation of gold nanoparticles was confirmed using optical spectra for characteristic surface plasmon band; the average size of gold nanoparticles was found to be 5-7 nm from electron microscopy measurements. The anti-tumor activity of prepared nanocomposite, vis-à-vis chloroquine itself, had been demonstrated using MCF-7 breast cancer cell line. To determine the binding affinity of gold-chloroquine nanocomposites to transport proteins present in blood serum, we studied the binding interaction of gold-chloroquine to bovine serum albumin (BSA), the most abundant plasma protein. The binding was studied by using isothermal titration calorimetry and fluorescence spectroscopy and was analyzed in terms of binding constant, entropy and enthalpy change. The gold-chloroquine nanocomposites were found to interact efficiently with BSA and fluorescence quenching experiments involving Trp212 suggests that the nanocomposites bind at site I of BSA.
Similar content being viewed by others
References
J. Gao, H. Gu and B. Xu, Acc. Chem. Res. 42, 1097–1107 (2009).
V. Dixit, J. Van den Bossche, D.M. Sherman, D.H. Thompson and R.P. Andres, Bioconjugate Chem. 17, 603–609 (2006).
C.C. You, A. Verma and V.M. Rotello, Soft Matter 2, 190–204 (2006).
J.A. Martinson, C.J. Montoya, X. Usuga, R. Ronquillo, A.L. Landay and S.N. Desai, Antimicrob. Agents Ch. 54, 871(2010).
M. Navarro, C. Hernandez, F. Vasquez, H. Goitia, L.E. Ojeda, M. Velasquez and G. Fraile, Transition Met. Chem. 33, 893–898 (2008).
A.R. Martirosyan, R. Rahim-Bata, A.B. Freeman, C.D. Clarke, R.L. Howard and J.S. Strobl, Biochem. Pharmacol. 68, 1729–1738 (2004).
K.H. Maclean, F.C. Dorsey, J.L. Cleveland and M.B. Kastan, J. Clin. Invest. 118, 79–88 (2008).
V.S. Jisha, K.T. Arun, M. Hariharan and D. Ramaiah, J. Am. Chem. Soc. 128, 6024–6025 (2006).
T. Peters Jr., Adv. Protein Chem. 37, 60065–60065 (1985).
X.M. He and D.C. Carter, Nature 358, 209–215 (1992).
J.R. Lakowicz, Principles of Fluorescence Spectroscopy. 3rd ed.: Springer: New York, 2006.
W.R. Ware, J. Phys. Chem. 66, 455–458 (1962).
P.D. Ross and S. Subramanian, Biochemistry 20, 3096–3102 (1981).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joshi, P., Chakraborti, S., Jaime, E.RV. et al. Anti-Tumor Chloroquine-Gold Nanocomposites and their Binding Interaction with Bovine Serum Albumin: Biophysical and Biochemical Aspects of Protein Binding. MRS Online Proceedings Library 1316, 929 (2011). https://doi.org/10.1557/opl.2011.595
Published:
DOI: https://doi.org/10.1557/opl.2011.595