Abstract
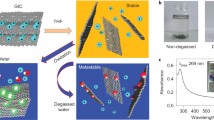
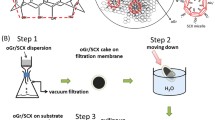
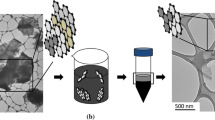
Graphene oxide (GO) is a nonstoichiometric two-dimensional material obtained from the chemical oxidation and exfoliation of graphite, which has recently attracted intense research interest as a precursor for bulk production of graphene. GO has long been believed to be hydrophilic due to its dispersibility in water. Recent work in our group, however, has found that GO is actually a two-dimensional amphiphile; the edge of the sheet-like material is hydrophilic, while the basal plane of the material contains more hydrophobic graphitic nanodomains. To prove the concept, we demonstrate GO’s surface activity at an air-water interface, as well as its utility in dispersing insoluble aromatic materials such as toluene, graphite, and carbon nanotubes in water. As a colloidal surfactant which can be converted to a conducting material, GO presents unique possibilities for aqueous solution processing of organic electronic materials.
Similar content being viewed by others
References
Cote, L. J., Kim, F., Huang, J., J. Am. Chem. Soc., 131, 1043 (2009).
Hummers, W. S. and Offeman, R. E., J. Am. Chem. Soc., 80, 1339 (1958).
Lerf, A., He, H.Y., Forster, M., Klinowski, J., J. Phys. Chem. B 102, 4477 (1998).
Gao, W., Alemany, L.B., Ci, L., Ajayan, P.M., Nat. Chem. 1, 403 (2009).
Dobelle, W. H., Beer, M., J. Cell Biol. 39, 733 (1968).
Stankovich, S., Dikin, D.A., Piner, R.D., Kohlhaas, K.A., Kleinhammes, A., Jia, Y., Wu, Y., Nguyen, S.T., Ruoff, R.S., Carbon 45, 1558 (2007).
Croft, R.C., Quarterly Rev. 14, 1 (1960).
Schniepp, H.C., Li, J.L., McAllister, M.J., Sai, H., Herrera-Alonso, M., Adamson, D.H., Prud’homme, R.K., Car, R., Saville, D.A., Aksay, I.A., J. Phys. Chem. B 110, 8535 (2006).
Cote, L.J., Cruz-Silva, R., Huang, J.. J. Am. Chem. Soc. 131, 11027 (2009).
Gilje, S, Dubin, S., Badakhshan, A., Farrar, J., Danczyk, S.A., Kaner, R.B., Adv. Mater. 22, 419 (2010).
Wang, Z., Zhou, X., Zhang, J., Boey, F., Zha, H., J Phys. Chem. C 113, 14071 (2009).
Ramesha, G.K., Sampath S., J. Phys. Chem. C 113, 7985 (2009).
Allen, M.J., Tung, V.C., Kaner, R.B., Chem. Rev. 110, 132 (2010).
Park, S., Ruoff R.S., Nat. Nanotechnol. 4, 217 (2009).
Compton, O.C., Nguyen, S.T., Small 6, 711 (2010).
Dikin, D.A., Stankovich, S., Zimney, E.J., Piner, R.D., Dommett, G.H.B., Evmenenko, G., Nguyen, S.T., Ruoff, R.S., Nature 448, 457 (2007).
Gilje, S., Han, S., Wang, M., Wang, K.L., Kaner, R.B., Nano Lett. 7, 3394 (2007).
Li, D., Kaner, R.B.. Science 320, 1170 (2008).
Li, D., Muller, M.B., Gilje, S., Kaner, R.B., Wallace, G.G., Nat. Nanotechnol. 3, 101 (2008).
Erickson K., Erni R., Lee Z., Alem N., Gannett W., Zettl A., Adv. Mater. 22, 4467 (2010).
Myers D., Surfactant Science and Technology, Wiley-Interscience, Hoboken, NJ (2006).
Lipp, M.M., Lee, K.Y.C., Zasadzinski, J.A., Waring, A.J., Rev. Sci. Instrum. 68, 2574 (1997).
Kim, J., Cote, L.J., Kim, F., Yuan, W., Shull, K.R., Huang, J., J. Am. Chem. Soc. 132, 8180 (2010).
Pickering, S.U., J. Chem. Soc. 91, 2001 (1907).
Islam, M.F., Rojas, E., Bergey, D.M., Johnson, A.T., Yodh, A.G., Nano Lett. 3, 269 (2003).
Moore, V.C., Strano, M.S., Haroz, E.H., Hauge, R.H., Smalley, R.E., Schmidt, J., Talmon, Y., Nano Lett. 3, 1379 (2003).
Grossiord, N., Loos, J., Regev, O., Koning, C.E., Chem. Mater. 18, 1089 (2006).
Vaisman, L., Wagner, H.D., Marom, G., Adv. Colloid Interface Sci. 128, 37 (2006).
Cote, L.J., Kim, J., Tung, V.C., Luo, J., Kim, F., Huang, J., Pure Appl. Chem. 83, 1, 95 (2011).
Luo, J., Cote, L.J., Tung, V.C., Tan, A.T.L., Goins, P.E., Wu, J., Huang, J., J. Am. Chem. Soc., 132, 17667 (2010).
Tung, V.C., Huang, J.H., Tevis, I., Kim, F., Kim, J., Chu, C.W., Stupp, S.I., Huang, J., J. Amer. Chem. Soc., 133, 4940 (2011).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Koltonow, A.R., Kim, J., Cote, L.J. et al. Graphene Oxide as a Two-dimensional Surfactant. MRS Online Proceedings Library 1344, 907 (2011). https://doi.org/10.1557/opl.2011.1367
Published:
DOI: https://doi.org/10.1557/opl.2011.1367