Abstract
Role of MOFs in CO2 chemical conversion; Photocatalytic and electrocatalytic CO2 reduction; Role of linkers and metals in CO2 chemical conversion; and MOF composites and films in CO2 conversion.
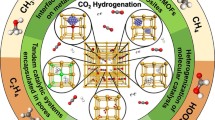
In this review, we analyze the emerging field of metal–organic frameworks (MOFs) as catalysts for chemical conversion of CO2, with examples ranging from heterogeneous CO2 organic transformation to heterogeneous CO2 hydrogenation, from photocatalytic to electrocatalytic CO2 reduction. We also discuss the role of MOF composites and films in CO2 transformation. Our goal is to have an instrument useful to identify the best MOFs for CO2 conversion.








Similar content being viewed by others
References
Chu S.: Carbon capture and sequestration. Science 325, 1599 (2009).
Lu A.H. and Hao G.P.: Porous materials for carbon dioxide capture. Annu. Rep. Prog. Chem. A 109, 484 (2013).
Tu W., Zhou Y., and Zou Z.: Photocatalytic conversion of CO2 into renewable hydrocarbon fuels: State-of-the-art accomplishment, challenges, and prospects. Adv. Mater. 26, 4607 (2014).
Qiao J., Liu Y., Hong F., and Zhang J.: A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 43, 631 (2014).
Rahmani F., Haghighi M., Estifaee P., and Rahimpour M.R.: A comparative study of two different membranes applied for auto-thermal methanol synthesis process. J. Nat. Gas Sci. Eng. 7, 60 (2012).
Yasuda H., He L.N., Sakakura T., and Hu C.: Efficient synthesis of cyclic carbonate from carbon dioxide catalyzed by polyoxometalate: The remarkable effects of metal substitution. J. Catal. 233, 119 (2005).
Hudson M.R., Queen W.L., Mason J.A., Fickel D.W., Lobo R.F., and Brown C.M.: Unconventional, highly selective CO2 adsorption in zeolite SSZ-13. J. Am. Chem. Soc. 134, 1970 (2012).
Zhang Y., Li B., Williams K., Gao W.Y., and Ma S.: A new microporous carbon material synthesized via thermolysis of a porous aromatic framework embedded with an extra carbon source for low-pressure CO2 uptake. Chem. Commun. 49, 10269 (2013).
Chughtai A.H., Ahmad N., Younus H.A., Laypkov A., and Verpoort F.: Metal-organic frameworks: Versatile heterogeneous catalysts for efficient catalytic organic transformations. Chem. Soc. Rev. 44, 6804 (2015).
Gascon J., Corma A., Kapteijn F., Llabrés I., and Xamena F.X.: Metal organic framework catalysis: Quo vadis? ACS Catal. 4, 361 (2014).
Nguyen P.T.K., Nguyen H.T.D., Nguyen H.N., Trickett C.A., Ton Q.T., Gutiérrez-Puebla E., Monge M.A., Cordova K.E., and Gándara F.: New metal-organic frameworks for chemical fixation of CO2. ACS Appl. Mater. Interfaces 10, 733 (2018).
Chen F., Dong T., Xu T., Li X., and Hu C.: Direct synthesis of cyclic carbonates from olefins and CO2 catalyzed by a MoO2(acac)2-quaternary ammonium salt system. Green Chem. 13, 2518 (2011).
Han Q., He C., Zhao M., Qi B., Niu J., and Duan C.: Engineering chiral polyoxometalate hybrid metal-organic frameworks for asymmetric dihydroxylation of olefins. J. Am. Chem. Soc. 135, 10186 (2013).
Zalomaeva O.V., Chibiryaev A.M., Kovalenko K.A., Kholdeeva O.A., Balzhinimaev B.S., and Fedin V.P.: Cyclic carbonates synthesis from epoxides and CO2 over metal-organic framework Cr-MIL-101. J. Catal. 298, 179 (2013).
Cho H.Y., Yang D.A., Kim J., Jeong S.Y., and Ahn W.S.: CO2 adsorption and catalytic application of Co-MOF-74 synthesized by microwave heating. Catal. Today 185, 35 (2012).
Guillerm V., Weseliński ŁJ, Belmabkhout Y., Cairns A.J., D'Elia V., Wojtas Ł, Adil K., and Eddaoudi M.: Discovery and introduction of a (3,18)-connected net as an ideal blueprint for the design of metal-organic frameworks. Nat. Chem. 6, 673 (2014).
Beyzavi M.H., Klet R.C., Tussupbayev S., Borycz J., Vermeulen N.A., Cramer C.J., Stoddart J.F., Hupp J.T., and Farha O.K.: A hafnium-based metal-organic framework as an efficient and multifunctional catalyst for facile CO2 fixation and regioselective and enantioretentive epoxide activation. J. Am. Chem. Soc. 136, 15861 (2014).
Zou R., Li P.-Z., Zeng Y.-F., Liu J., Zhao R., Duan H., Luo Z., Wang J.-G., Zou R., and Zhao Y.: Bimetallic metal-organic frameworks: Probing the lewis acid site for CO2 conversion. Small 12, 2334 (2016).
Wang S., Yang L., He G., Shi B., Li Y., Wu H., Zhang R., Nunes S., and Jiang Z.: Two-dimensional nanochannel membranes for molecular and ionic separations. Chem. Soc. Rev. 49, 1071 (2020).
Gao W.Y., Chen Y., Niu Y., Williams K., Cash L., Perez P.J., Wojtas L., Cai J., Chen Y.S., and Ma S.: Crystal engineering of an nbo topology metal-organic framework for chemical fixation of CO2 under ambient conditions. Angew. Chem. Int. Ed. 53, 2615 (2014).
Ma D., Li B., Liu K., Zhang X., Zou W., Yang Y., Li G., Shi Z., and Feng S.: Bifunctional MOF heterogeneous catalysts based on the synergy of dual functional sites for efficient conversion of CO2 under mild and co-catalyst free conditions. J. Mater. Chem. A 3, 23136 (2015).
Zhou Z., He C., Xiu J., Yang L., and Duan C.: Metal-organic polymers containing discrete single-walled nanotube as a heterogeneous catalyst for the cycloaddition of carbon dioxide to epoxides. J. Am. Chem. Soc. 137, 15066 (2015).
Gao W.Y., Chen Y., Niu Y., Williams K., Cash L., Perez P.J., Wojtas L., Cai J., Chen Y.S., and Ma S.: Crystal engineering of an nbo topology metal-organic framework for chemical fixation of CO2 under ambient conditions. Angew. Chem. Int. Ed. 53, 2615 (2014).
Kathalikkattil A.C., Roshan R., Tharun J., Babu R., Jeong G.S., Kim D.W., Cho S.J., and Park D.W.: A sustainable protocol for the facile synthesis of zinc-glutamate MOF: An efficient catalyst for room temperature CO2 fixation reactions under wet conditions. Chem. Commun. 52, 280 (2016).
Rachuri Y., Kurisingal J.F., Chitumalla R.K., Vuppala S., Gu Y., Jang J., Choe Y., Suresh E., and Park D.W.: Adenine-based Zn(II)/Cd(II) metal-organic frameworks as efficient heterogeneous catalysts for facile CO2 fixation into cyclic carbonates: A DFT-supported study of the reaction mechanism. Inorg. Chem. 58, 11389 (2019).
Miralda C.M., MacIas E.E., Zhu M., Ratnasamy P., and Carreon M.A.: Zeolitic imidazole framework-8 catalysts in the conversion of CO2 to chloropropene carbonate. ACS Catal. 2, 180 (2012).
Kim Y.J. and Park D.W.: Functionalized IRMOF-3: An efficient heterogeneous catalyst for the cycloaddition of allyl glycidyl ether and CO2. J. Nanosci. Nanotechnol. 13, 2307 (2013).
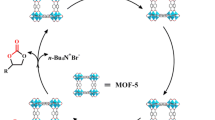
Song J., Zhang Z., Hu S., Wu T., Jiang T., and Han B.: MOF-5/n-Bu4NBr: An efficient catalyst system for the synthesis of cyclic carbonates from epoxides and CO2 under mild conditions. Green Chem. 11, 1031 (2009).
Zhang L., Yuan S., Feng L., Guo B., Qin J.-S., Xu B., Lollar C., Sun D., and Zhou H.-C.: Pore-environment engineering with multiple metal sites in rare-earth porphyrinic metal-organic frameworks. Angew. Chem. Int. Ed. 57, 5095 (2018).
Ji X.H., Zhu N.N., Ma J.G., and Cheng P.: Conversion of CO2 into cyclic carbonates by a Co(ii) metal-organic framework and the improvement of catalytic activity: Via nanocrystallization. Dalt. Trans. 47, 1768 (2018).
Feng D., Chung W.C., Wei Z., Gu Z.Y., Jiang H.L., Chen Y.P., Darensbourg D.J., and Zhou H.C.: Construction of ultrastable porphyrin Zr metal-organic frameworks through linker elimination. J. Am. Chem. Soc. 135, 17105 (2013).
Gao W.-Y., Chen Y., Niu Y., Williams K., Cash L., Perez P.J., Wojtas L., Cai J., Chen Y.-S., and Ma S.: Crystal engineering of an nbo topology metal-organic framework for chemical fixation of CO2 under ambient conditions. Angew. Chem. Int. Ed. 53, 2615 (2014).
Kleist W., Jutz F., Maciejewski M., and Baiker A.: Mixed-linker metal-organic frameworks as catalysts for the synthesis of propylene carbonate from propylene oxide and CO2. Eur. J. Inorg. Chem. 2009, 3552 (2009).
Lescouet T., Chizallet C., and Farrusseng D.: The origin of the activity of amine-functionalized metal-organic frameworks in the catalytic synthesis of cyclic carbonates from epoxide and CO2. ChemCatChem 4, 1725 (2012).
Kim J., Kim S.N., Jang H.G., Seo G., and Ahn W.S.: CO2 cycloaddition of styrene oxide over MOF catalysts. Appl. Catal. A Gen. 453, 175 (2013).
Beyzavi M.H., Stephenson C.J., Liu Y., Karagiaridi O., Hupp J.T., and Farha O.K.: Metal-organic framework-based catalysts: Chemical fixation of CO2 with epoxides leading to cyclic organic carbonates. Front. Energy Res. 3, 63 (2015).
Vismara R., Tuci G., Mosca N., Domasevitch K.V., Di Nicola C., Pettinari C., Giambastiani G., Galli S., and Rossin A.: Amino-decorated bis(pyrazolate) metal–organic frameworks for carbon dioxide capture and green conversion into cyclic carbonates. Inorg. Chem. Front. 6, 533 (2019).
Müller P., Bucior B., Tuci G., Luconi L., Getzschmann J., Kaskel S., Snurr R.Q., Giambastiani G., and Rossin A.: Computational screening, synthesis and testing of metal-organic frameworks with a bithiazole linker for carbon dioxide capture and its green conversion into cyclic carbonates. Mol. Syst. Des. Eng. 4, 1000 (2019).
Babu R., Kathalikkattil A.C., Roshan R., Tharun J., Kim D.W., and Park D.W.: Dual-porous metal organic framework for room temperature CO2 fixation via cyclic carbonate synthesis. Green Chem. 18, 232 (2015).
Cao C.S., Shi Y., Xu H., and Zhao B.: A multifunctional MOF as a recyclable catalyst for the fixation of CO2 with aziridines or epoxides and as a luminescent probe of Cr(VI). Dalt. Trans. 47, 4545 (2018).
Kumar S., Verma G., Gao W.-Y., Niu Z., Wojtas L., and Ma S.: Anionic metal-organic framework for selective dye removal and CO2 fixation. Eur. J. Inorg. Chem. 2016, 4373 (2016).
Li X.Y., Ma L.N., Liu Y., Hou L., Wang Y.Y., and Zhu Z.: Honeycomb metal-organic framework with lewis acidic and basic bifunctional sites: Selective adsorption and CO2 catalytic fixation. ACS Appl. Mater. Interfaces 10, 10965 (2018).
He H., Sun Q., Gao W., Perman J.A., Sun F., Zhu G., Aguila B., Forrest K., Space B., and Ma S.: A stable metal-organic framework featuring a local buffer environment for carbon dioxide fixation. Angew. Chem. Int. Ed. 57, 4657 (2018).
Ansari S.N., Kumar P., Gupta A.K., Mathur P., and Mobin S.M.: Catalytic CO2 fixation over a robust lactam-functionalized Cu(II) metal organic framework. Inorg. Chem. 58, 9723 (2019).
Liang L., Liu C., Jiang F., Chen Q., Zhang L., Xue H., Jiang H.L., Qian J., Yuan D., and Hong M.: Carbon dioxide capture and conversion by an acid-base resistant metal-organic framework. Nat. Commun. 8, 1 (2017).
Zhou X., Zhang Y., Yang X., Zhao L., and Wang G.: Functionalized IRMOF-3 as efficient heterogeneous catalyst for the synthesis of cyclic carbonates. J. Mol. Catal. A Chem. 361–362, 12 (2012).
Tharun J., Bhin K.M., Roshan R., Kim D.W., Kathalikkattil A.C., Babu R., Ahn H.Y., Won Y.S., and Park D.W.: Ionic liquid tethered post functionalized ZIF-90 framework for the cycloaddition of propylene oxide and CO2. Green Chem. 18, 2479 (2016).
Kaneti Y.V., Dutta S., Hossain M.S.A., Shiddiky M.J.A., Tung K.L., Shieh F.K., Tsung C.K., Wu K.C.W., and Yamauchi Y.: Strategies for improving the functionality of zeolitic imidazolate frameworks: Tailoring nanoarchitectures for functional applications. Adv. Mater. 29, 1700213 (2017).
Bhin K.M., Tharun J., Roshan K.R., Kim D.W., Chung Y., and Park D.W.: Catalytic performance of zeolitic imidazolate framework ZIF-95 for the solventless synthesis of cyclic carbonates from CO2 and epoxides. J. CO2 Util. 17, 112 (2017).
Liang J., Xie Y.Q., Wang X.S., Wang Q., Liu T.T., Huang Y.B., and Cao R.: An imidazolium-functionalized mesoporous cationic metal-organic framework for cooperative CO2 fixation into cyclic carbonate. Chem. Commun. 54, 342 (2018).
Manjolinho F., Arndt M., Gooßen K., and Gooßen L.J.: Catalytic C-H carboxylation of terminal alkynes with carbon dioxide. ACS Catal. 2, 2014 (2012).
Xiong G., Yu B., Dong J., Shi Y., Zhao B., and He L.N.: Cluster-based MOFs with accelerated chemical conversion of CO2 through C-C bond formation. Chem. Commun. 53, 6013 (2017).
Zhang Y., Li B., Krishna R., Wu Z., Ma D., Shi Z., Pham T., Forrest K., Space B., and Ma S.: Highly selective adsorption of ethylene over ethane in a MOF featuring the combination of open metal site and π-complexation. Chem. Commun. 51, 2714 (2015).
Li B., Zhang Y., Krishna R., Yao K., Han Y., Wu Z., Ma D., Shi Z., Pham T., Space B., Liu J., Thallapally P.K., Liu J., Chrzanowski M., and Ma S.: Introduction of π-complexation into porous aromatic framework for highly selective adsorption of ethylene over ethane. J. Am. Chem. Soc. 136, 8654 (2014).
Zhou Z., He C., Yang L., Wang Y., Liu T., and Duan C.: Alkyne activation by a porous silver coordination polymer for heterogeneous catalysis of carbon dioxide cycloaddition. ACS Catal. 7, 2248 (2017).
Zhang G., Yang H., and Fei H.: Unusual missing linkers in an organosulfonate-based primitive-cubic (pcu)-type metal-organic framework for CO2 capture and conversion under ambient conditions. ACS Catal. 8, 2519 (2018).
Liu X.H., Ma J.G., Niu Z., Yang G.M., and Cheng P.: An efficient nanoscale heterogeneous catalyst for the capture and conversion of carbon dioxide at ambient pressure. Angew. Chem. Int. Ed. 54, 988 (2015).
Molla R.A., Ghosh K., Banerjee B., Iqubal M.A., Kundu S.K., Islam S.M., and Bhaumik A.: Silver nanoparticles embedded over porous metal organic frameworks for carbon dioxide fixation via carboxylation of terminal alkynes at ambient pressure. J. Colloid Interface Sci. 477, 220 (2016).
Gao W.Y., Wu H., Leng K., Sun Y., and Ma S.: Inserting CO2 into Aryl C-H bonds of metal-organic frameworks: CO2 Utilization for direct heterogeneous C-H activation. Angew. Chem. Int. Ed. 55, 5472 (2016).
McDonald T.M., Mason J.A., Kong X., Bloch E.D., Gygi D., Dani A., Crocellà V., Giordanino F., Odoh S.O., Drisdell W.S., Vlaisavljevich B., Dzubak A.L., Poloni R., Schnell S.K., Planas N., Lee K., Pascal T., Wan L.F., Prendergast D., Neaton J. B., Smit B., Kortright Gagliardi, L. Bordiga S. Reimer J.A., Long J.R.: Cooperative insertion of CO2 in diamine-appended metal-organic frameworks. Nature 519, 303 (2015).
Zhao D., Liu X.-H., Zhu C., Kang Y.-S., Wang P., Shi Z., Lu Y., and Sun W.-Y.: Efficient and reusable metal-organic framework catalysts for carboxylative cyclization of propargylamines with carbon dioxide. ChemCatChem 9, 4598 (2017).
Li W., Wang H., Jiang X., Zhu J., Liu Z., Guo X., and Song C.: A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts. RSC Adv. 8, 7651 (2018).
Younas M., Loong Kong L., Bashir M.J.K., Nadeem H., Shehzad A., and Sethupathi S.: Recent advancements, fundamental challenges, and opportunities in catalytic methanation of CO2. Energy Fuels 30, 8815 (2016).
Zhen W., Li B., Lu G., and Ma J.: Enhancing catalytic activity and stability for CO2 methanation on Ni@MOF-5 via control of active species dispersion. Chem. Commun. 51, 1728 (2015).
Li W., Zhang A., Jiang X., Chen C., Liu Z., Song C., and Guo X.: Low temperature CO2 methanation: ZIF-67-derived Co-based porous carbon catalysts with controlled crystal morphology and size. ACS Sustain. Chem. Eng. 5, 7824 (2017).
Zhan G. and Zeng H.C.: ZIF-67-derived nanoreactors for controlling product selectivity in CO2 hydrogenation. ACS Catal. 7, 7509 (2017).
Lippi R., Howard S.C., Barron H., Easton C.D., Madsen I.C., Waddington L.J., Vogt C., Hill M.R., Sumby C.J., Doonan C.J., and Kennedy D.F.: Highly active catalyst for CO2 methanation derived from a metal organic framework template. J. Mater. Chem. A 5, 12990 (2017).
Zhang T., Manna K., and Lin W.: Metal-organic frameworks stabilize solution-inaccessible cobalt catalysts for highly efficient broad-scope organic transformations. J. Am. Chem. Soc. 138, 3241 (2016).
Yin Y., Hu B., Li X., Zhou X., Hong X., and Liu G.: Pd@zeolitic imidazolate framework-8 derived PdZn alloy catalysts for efficient hydrogenation of CO2 to methanol. Appl. Catal. B Environ. 234, 143 (2018).
Rungtaweevoranit B., Baek J., Araujo J.R., Archanjo B.S., Choi K.M., Yaghi O.M., and Somorjai G.A.: Copper nanocrystals encapsulated in Zr-based metal-organic frameworks for highly selective CO2 hydrogenation to methanol. Nano Lett. 16, 7645 (2016).
An B., Zhang J., Cheng K., Ji P., Wang C., and Lin W.: Confinement of ultrasmall Cu/ZnOx nanoparticles in metal-organic frameworks for selective methanol synthesis from catalytic hydrogenation of CO2. J. Am. Chem. Soc. 139, 3834 (2017).
An B., Li Z., Song Y., Zhang J., Zeng L., Wang C., and Lin W.: Cooperative copper centres in a metal–organic framework for selective conversion of CO2 to ethanol. Nat. Catal. 2, 709 (2019).
Liu J., Sun Y., Jiang X., Zhang A., Song C., and Guo X.: Pyrolyzing ZIF-8 to N-doped porous carbon facilitated by iron and potassium for CO2 hydrogenation to value-added hydrocarbons. J. CO2 Util. 25, 120 (2018).
Liu J., Zhang A., Liu M., Hu S., Ding F., Song C., and Guo X.: Fe-MOF-derived highly active catalysts for carbon dioxide hydrogenation to valuable hydrocarbons. J. CO2 Util. 21, 100 (2017).
An B., Cheng K., Wang C., Wang Y., and Lin W.: Pyrolysis of metal-organic frameworks to Fe3O4@Fe5C2 core-shell nanoparticles for fischer-tropsch synthesis. ACS Catal. 6, 3610 (2016).
Meng W., Chen W., Zhao L., Huan G.Y., Zhu M., Huang Y., Fu Y., Geng F., Yu J., Chen X., and Zhi C.: Porous Fe3O4/carbon composite electrode material prepared from metal-organic framework template and effect of temperature on its capacitance. Nano Energy 8, 133 (2014).
Li Z., Rayder T.M., Luo L., Byers J.A., and Tsung C.K.: Aperture-opening encapsulation of a transition metal catalyst in a metal-organic framework for CO2 hydrogenation. J. Am. Chem. Soc. 140, 8082 (2018).
Xu W., Zhang X., Dong M., Zhao J., and Di L.: Plasma-assisted Ru/Zr-MOF catalyst for hydrogenation of CO2 to methane. Plasma Sci. Technol. 21, 044004 (2019).
Wang C., Xie Z., Dekrafft K.E., and Lin W.: Doping metal-organic frameworks for water oxidation, carbon dioxide reduction, and organic photocatalysis. J. Am. Chem. Soc. 133, 13445 (2011).
Alvaro M., Carbonell E., Ferrer B., Llabrés I., Xamena F.X., and Garcia H.: Semiconductor behavior of a metal-organic framework (MOF). Chemistry 13, 5106 (2007).
Lee Y., Kim S., Kang J.K., and Cohen S.M.: Photocatalytic CO2 reduction by a mixed metal (Zr/Ti), mixed ligand metal-organic framework under visible light irradiation. Chem. Commun. 51, 5735 (2015).
Nasalevich M.A., Hendon C.H., Santaclara J.G., Svane K., Van Der Linden B., Veber S.L., Fedin M.V., Houtepen A.J., Van Der Veen M.A., Kapteijn F., Walsh A., and Gascon J.: Electronic origins of photocatalytic activity in d0 metal organic frameworks. Sci. Rep. 6, 23676 (2016).
Windle C.D., George M.W., Perutz R.N., Summers P.A., Sun X.Z., and Whitwood A.C.: Comparison of rhenium-porphyrin dyads for CO2 photoreduction: Photocatalytic studies and charge separation dynamics studied by time-resolved IR spectroscopy. Chem. Sci. 6, 6847 (2015).
Fu Y., Sun D., Chen Y., Huang R., Ding Z., Fu X., and Li Z.: An amine-functionalized titanium metal-organic framework photocatalyst with visible-light-induced activity for CO2 reduction. Angew. Chem. Int. Ed. 51, 3364 (2012).
Cavka J.H., Jakobsen S., Olsbye U., Guillou N., Lamberti C., Bordiga S., and Lillerud K.P.: A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 130, 13850 (2008).
Wang Y., Huang N.Y., Shen J.Q., Liao P.Q., Chen X.M., and Zhang J.P.: Hydroxide ligands cooperate with catalytic centers in metal-organic frameworks for efficient photocatalytic CO2 reduction. J. Am. Chem. Soc. 140, 38 (2018).
Kajiwara T., Fujii M., Tsujimoto M., Kobayashi K., Higuchi M., Tanaka K., and Kitagawa S.: Photochemical reduction of low concentrations of CO2 in a porous coordination polymer with a ruthenium(II)-CO complex. Angew. Chem. Int. Ed. 55, 2697 (2016).
Wang D., Huang R., Liu W., Sun D., and Li Z.: Fe-based MOFs for photocatalytic CO2 reduction: Role of coordination unsaturated sites and dual excitation pathways. ACS Catal. 4, 4254 (2014).
Dao X.Y., Guo J.H., Wei Y.P., Guo F., Liu Y., and Sun W.Y.: Solvent-free photoreduction of CO2 to CO catalyzed by Fe-MOFs with superior selectivity. Inorg. Chem. 58, 8517 (2019).
Xu H.Q., Hu J., Wang D., Li Z., Zhang Q., Luo Y., Yu S.H., and Jiang H.L.: Visible-light photoreduction of CO2 in a metal-organic framework: Boosting electron-hole separation via electron trap states. J. Am. Chem. Soc. 137, 13440 (2015).
Chen D., Xing H., Wang C., and Su Z.: Highly efficient visible-light-driven CO2 reduction to formate by a new anthracene-based zirconium MOF via dual catalytic routes. J. Mater. Chem. A 4, 2657 (2016).
Wu L.Y., Mu Y.F., Guo X.X., Zhang W., Zhang Z.M., Zhang M., and Lu T.B.: Encapsulating perovskite quantum dots in iron-based Metal–Organic Frameworks (MOFs) for efficient photocatalytic CO2 reduction. Angew. Chem. Int. Ed. 58, 9491 (2019).
Zhu W., Zhang C., Li Q., Xiong L., Chen R., Wan X., Wang Z., Chen W., Deng Z., and Peng Y.: Selective reduction of CO2 by conductive MOF nanosheets as an efficient co-catalyst under visible light illumination. Appl. Catal. B Environ. 238, 339 (2018).
Al-Omari A.A., Yamani Z.H., and Nguyen H.L.: Electrocatalytic CO2 reduction: From homogeneous catalysts to heterogeneous-based reticular chemistry. Molecules 23, 2835 (2018).
Huang Z., Dong P., Zhang Y., Nie X., Wang X., and Zhang X.: A ZIF-8 decorated TiO2 grid-like film with high CO2 adsorption for CO2 photoreduction. J. CO2 Util. 24, 369 (2018).
Liu Q., Low Z.X., Li L., Razmjou A., Wang K., Yao J., and Wang H.: ZIF-8/Zn2GeO4 nanorods with an enhanced CO2 adsorption property in an aqueous medium for photocatalytic synthesis of liquid fuel. J. Mater. Chem. A 1, 11563 (2013).
Habisreutinger S.N., Schmidt-Mende L., and Stolarczyk J.K.: Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Ed. 52, 7372 (2013).
Sun D., Liu W., Fu Y., Fang Z., Sun F., Fu X., Zhang Y., and Li Z.: Noble metals can have different effects on photocatalysis over metal-organic frameworks (MOFs): A case study on M/NH2-MIL-125(Ti) (M = Pt and Au). Chem. A Eur. J. 20, 4780 (2014).
Wang S., Lin J., and Wang X.: Semiconductor-redox catalysis promoted by metal-organic frameworks for CO2 reduction. Phys. Chem. Chem. Phys. 16, 14656 (2014).
Shi L., Wang T., Zhang H., Chang K., and Ye J.: Electrostatic self-assembly of nanosized carbon nitride nanosheet onto a zirconium metal-organic framework for enhanced photocatalytic CO2 reduction. Adv. Funct. Mater. 25, 5360 (2015).
Ye L., Liu J., Gao Y., Gong C., Addicoat M., Heine T., Wöll C., and Sun L.: Highly oriented MOF thin film-based electrocatalytic device for the reduction of CO2 to CO exhibiting high faradaic efficiency. J. Mater. Chem. A 4, 15320 (2016).
Hod I., Sampson M.D., Deria P., Kubiak C.P., Farha O.K., and Hupp J.T.: Fe-porphyrin-based metal-organic framework films as high-surface concentration, heterogeneous catalysts for electrochemical reduction of CO2. ACS Catal. 5, 6302 (2015).
Shao P., Yi L., Chen S., Zhou T., and Zhang J.: Metal-organic frameworks for electrochemical reduction of carbon dioxide: The role of metal centers. J. Energy Chem. 40, 156 (2020).
Zhang H., Li J., Tan Q., Lu L., Wang Z., and Wu G.: Metal–organic frameworks and their derived materials as electrocatalysts and photocatalysts for CO2 reduction: Progress, challenges, and perspectives. Chem. A Eur. J. 24, 18137 (2018).
Lei Z., Xue Y., Chen W., Qiu W., Zhang Y., Horike S., and Tang L.: MOFs-based heterogeneous catalysts: New opportunities for energy-related CO2 conversion. Adv. Energy Mater. 8, 1801587 (2018).
Zhang X., Wu Z., Zhang X., Li L., Li Y., Xu H., Li X., Yu X., Zhang Z., Liang Y., and Wang H.: Highly selective and active CO2 reduction electrocatalysts based on cobalt phthalocyanine/carbon nanotube hybrid structures. Nat. Commun. 8, 1 (2017).
Qiu Y.L., Zhong H.X., Zhang T.T., Xu W.B., Su P.P., Li X.F., and Zhang H.M.: Selective electrochemical reduction of carbon dioxide using Cu based metal organic framework for CO2 capture. ACS Appl. Mater. Interfaces 10, 2480 (2018).
Jiang X., Wu H., Chang S., Si R., Miao S., Huang W., Li Y., Wang G., and Bao X.: Boosting CO2 electroreduction over layered zeolitic imidazolate frameworks decorated with Ag2O nanoparticles. J. Mater. Chem. A 5, 19371 (2017).
Huan T.N., Ranjbar N., Rousse G., Sougrati M., Zitolo A., Mougel V., Jaouen F., and Fontecave M.: Electrochemical reduction of CO2 catalyzed by Fe-N-C materials: A structure-selectivity study. ACS Catal. 7, 1520 (2017).
Nam D.H., Bushuyev O.S., Li J., De Luna P., Seifitokaldani A., Dinh C.T., De García Arquer F.P., Wang Y., Liang Z., Proppe A.H., Tan C.S., Todorović P., Shekhah O., Gabardo C.M., Jo J.W., Choi J., Choi M.J., Baek S.W., et al.: Metal-organic frameworks mediate Cu coordination for selective CO2 electroreduction. J. Am. Chem. Soc. 140, 11378 (2018).
Kornienko N., Zhao Y., Kley C.S., Zhu C., Kim D., Lin S., Chang C.J., Yaghi O.M., and Yang P.: Metal-organic frameworks for electrocatalytic reduction of carbon dioxide. J. Am. Chem. Soc. 137, 14129 (2015).
Kang X., Zhu Q., Sun X., Hu J., Zhang J., Liu Z., and Han B.: Highly efficient electrochemical reduction of CO2 to CH4 in an ionic liquid using a metal-organic framework cathode. Chem. Sci. 7, 266 (2016).
Rosen B.A., Haan J.L., Mukherjee P., Braunschweig B., Zhu W., Salehi-Khojin A., Dlott D.D., and Masel R.I.: In situ spectroscopic examination of a low overpotential pathway for carbon dioxide conversion to carbon monoxide. J. Phys. Chem. C 116, 15307 (2012).
Senthil Kumar R., Senthil Kumar S., and Anbu Kulandainathan M.: Highly selective electrochemical reduction of carbon dioxide using Cu based metal organic framework as an electrocatalyst. Electrochem. Commun. 25, 70 (2012).
Hinogami R., Yotsuhashi S., Deguchi M., Zenitani Y., Hashiba H., and Yamada Y.: Electrochemical reduction of carbon dioxide using a copper rubeanate metal organic framework. ECS Electrochem. Lett. 1, H17 (2012).
Albo J., Vallejo D., Beobide G., Castillo O., Castaño P., and Irabien A.: Copper-based metal–organic porous materials for CO2 electrocatalytic reduction to alcohols. ChemSusChem 10, 1100 (2017).
Author information
Authors and Affiliations
Corresponding author
Abbreviations
- [(Etim-H2BDC)Br]
-
2-(3-Ethylimidazol-1-yl)terephthalic acid
- BDC
-
1,4-Benzenedicarboxylate
- bpdc
-
Biphenyl-4,4′-dicarboxylate
- bpy
-
4,4′-Bipyridine
- BPYDC
-
2,2′-Bipyridine-5,5′-dicarboxylate
- BPZNH2
-
3-Amino-4,4′-bipyrazolate
- Btz
-
1,5-Bis(5-tetrazole)-3-oxapentanoate
- CCU
-
Carbon Capture and Utilization
- dabco
-
1,4-Diazabicyclooctane
- dcppy
-
2-Phenylpyridine-5,4′-dicarboxylate
- DBU
-
1,8-Diazabicyclo-[5.4.0]underc-7-ene
- DMF
-
N,N-Dimetilformammide
- ECH
-
Epichloridrine
- FTO
-
Fluorine-doped tin oxide
- FTS
-
Fischer Tropsch Catalysis
- IN
-
Isonicotinate
- L1
-
10-(4-Carboxy-phenyl)-10H-phenoxazine- 3,6-dicarboxylic)
- L2
-
N1-(4-(1H-Imidazol-1-yl)benzyl)-N1-(2-aminoethyl)-ethane- 1,2-diamine)
- L3
-
2,2′-Bipyridine-5,5′-dicarboxylate
- NPs
-
Nanoparticles
- OCs
-
Cyclic organic carbonates
- tBuPNP
-
2,6-Bis((di-tert-butyl-phosphino)methyl)pyridine
- SURMOF
-
Surface-Anchored Metal–Organic Frameworks
- TATAB
-
4,4′,4′′-((1,3,5-Triazine-2,4,6-triyl)-tris(azanediyl))-tribenzoic acid
- TBAB
-
Tetrabutylammonium bromide
- TCPE
-
Tetrakis(4-carboxyphenyl)ethylene
- TCPP
-
[Meso-tetra(4-carboxyphenyl)porphyrin]
- TEOA
-
Triethanolamine
- TzTz
-
[2,2′-Bithiazole]-5,5′-dicarboxylate
- TOF
-
Turnover frequency
- TON
-
Total turnover number
- ZIF
-
Zeolitic imidazolate framework
- Ba-MOF
-
[Ba2(BDPO)(H2O)]
- Co-MOF
-
[Co(μ3-L)(H2O)]⋅0.5H2O (L = thiazolidine 2,4-dicarboxylate)
- Cr-MIL-101
-
Cr3O(H2O)2F(BDC)3
- Fe-MIL-88B
-
Fe3O(solvent)3Cl(BDC)3(solvent)m
- Fe-MOF-525
-
Zr6O4(OH)8(TCPP)2
- Hf-NU-1000
-
Hf6(OH)16(TBAPy)2
- HKUST-1
-
[Cu3(BTC)2]
- IRMOF-3
-
[Zn4O(NH2-BDC)3]
- JUC-1000
-
[Cu24(BDPO)12(H2O)12]·30DMF·14H2O
- MIL-101
-
Cr3O(H2O)2F(BDC)3
- MIL-125
-
Ti8O8(OH)4(BDC)6
- MMCF-2
-
[Cu2(Cu-tactmb)(H2O)3(NO3)2]
- MMPF-9
-
Cu6(Cu-tetrakis(3,5-dicarboxybiphenyl) porphinate)O4(HCOO)4
- MOF-505
-
Cu2(EBTC)(H2O)2
- MOF-525
-
Zr6O4(OH)8(TCPP)2
- NH2-MIL-125
-
[Ti8O8(OH)4(NH2-BDC)6]
- Ni-TCPE-1
-
{[Ni4(TCPE)(μ3-OH)2(H2O)6]⋅2(H2O)⋅DMA}n
- NNU-28
-
[Zr6O4(OH)4(L)6]⋅6DMF (L = 4,40-(anthracene- 9,10-diylbis(ethyne-2,1-diyl))dibenzoate)
- PCN-222
-
Zr6(μ3-OH)8(OH)8-(TCPP)2
- PCN-900
-
[(CH3)2NH2]2[Eu6(μ3-OH)8(TCPP)1.5 (DCDPS)3]·(solvent)x
- UiO-66
-
Zr6O4(OH)4(BDC)6
- UiO-67
-
Zr6(μ3-O)4(μ3-OH)4(BPDC)12
- ZIF-8
-
Zn(MeIm)2
- ZIF-9
-
Co(PhIm)2
- Zn-btz-MOF
-
Zn(2-(2-hydroxyphenyl)benzothiazolate)2
- Zr-NU-1000
-
Zr6(OH)16(TBAPy)2
Rights and permissions
About this article
Cite this article
Pettinari, C., Tombesi, A. Metal–organic frameworks for chemical conversion of carbon dioxide. MRS Energy & Sustainability 7, 31 (2020). https://doi.org/10.1557/mre.2020.35
Received:
Accepted:
Published:
DOI: https://doi.org/10.1557/mre.2020.35