Abstract
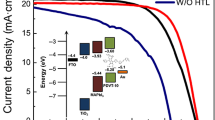
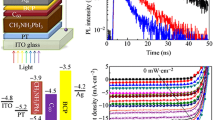
Direct arylation polycondensation (DArP) is an emerging synthetic method of producing conjugated polymers in an environmentally benign and cost-effective manner. We now report the synthesis of hole-transporting conjugated polymers, namely, DPP-OMe (Mn= 7.9 kg/mol) and DPP-F (Mn = 12.6 kg/mol), under microwave-assisted DArP conditions. These two polymers and the previously synthesized 3,6-Cbz-EDOT were evaluated as hole-transporting materials in mesoscopic perovskite solar cells. 3,6-Cbz-EDOT synthesized by DArP exhibited higher hole mobility and better photovoltaic properties than that synthesized by the Stille polycondensation. Moreover, chemical dopants improved the short-circuit current density (Jsc) and fill factor.








Similar content being viewed by others
References
Y. Shirota and H. Kageyama: Charge carrier transporting molecular materials and their applications in devices. Chem. Rev. 107, 953 (2007).
J. Wang, K. Liu, L. Ma, and X. Zhan: Triarylamine: versatile platform for organic, dye-sensitized, and perovskite solar cells. Chem. Rev. 116, 14675 (2016).
L. Calió, S. Kazim, M. Grätzel, and S. Ahmad: Hole-transporting materials for perovskite solar cells. Angew. Chem. Int. Ed. 55, 14522 (2016).
S. Ameen, M.A. Rub, S.A. Kosa, K.A. Alamry, M.S. Akhtar, H.-S. Shin, H.-K. Seo, A.M. Asiri, and M.K. Nazeeruddin: Perovskite solar cells: influence of hole transporting materials on power conversion efficiency. ChemSusChem. 9, 10 (2016).
P. Agarwala and D. Kabra: A review on triphenylamine (TPA) based organic hole transport materials (HTMs) for dye sensitized solar cells (DSSCs) and perovskite solar cells (PSCs): evolution and molecular engineering. J. Mater. Chem. A 5, 1348 (2017).
A. Krishna and A.C. Grimsdale: Hole transporting materials for mesoscopic perovskite solar cells—towards a rational design? J. Mater. Chem. A 5, 16446 (2017).
C. Rodríguez-Seco, L. Cabau, A. Vidal-Ferran, and E. Palomares: Advances in the synthesis of small molecules as hole transport materials for lead halide perovskite solar cells. Acc. Chem. Res. 51, 869 (2018).
W. Zhou, Z. Wen, and P. Gao: Less is more: dopant-free hole transporting materials for high-efficiency perovskite solar cells. Adv. Energy Mater. 8, 1702512 (2018).
Y. Tao, C. Yang, and J. Qin: Organic host materials for phosphorescent organic light-emitting diodes. Chem. Soc. Rev. 40, 2943 (2011).
J. Veres, S.D. Ogier, S.W. Leeming, D.C. Cupertino, and S.M. Khaffaf: Low-k insulators as the choice of dielectrics in organic field-effect transistors. Adv. Funct. Mater. 13, 199 (2003).
M. Horie, Y. Luo, J.J. Morrison, L.Z. Majewski, A. Song, B.R. Saunders, and M.L. Turner: Triarylamine polymers by microwave-assisted polycondensation for use in organic field-effect transistors. J. Mater. Chem. 18, 5230 (2008).
T. Michinobu, H. Kumazawa, E. Otsuki, H. Usui, and K. Shigehara: Synthesis and properties of nitrogen-linked poly(2,7-carbazole)s as hole-transport material for organic light emitting materials. J. Polym. Sci. Part A 47, 3880 (2009).
K. Tsuchiya, T. Shimomura, and K. Ogino: Preparation of diblock copolymer based on poly(4-n-butyltriphenylamine) via palladium coupling polymerization. Polymer 50, 95 (2009).
K. Tsuchiya, T. Sakakura, and K. Ogino: Synthesis of triphenylamine copolymers and effect of their chemical structures on physical properties. Macromolecules 44, 5200 (2011).
L.-T. Huang, H.-J. Yen, and G.-S. Liou: Substituent effect on electrochemical and electrochromic behaviors of ambipolar aromatic polyimides based on aniline derivatives. Macromolecules 44, 9595 (2011).
A. Iwan and D. Sek: Polymers with triphenylamine units: photonic and electroactive materials. Prog. Polym. Sci. 36, 1277 (2011).
Y.-G. Ko, W. Kwon, H.-J. Yen, C.-W. Chang, D.M. Kim, K. Kim, S.G. Hahm, T.J. Lee, G.-S. Liou, and M. Ree: Various digital memory behaviors of functional aromatic polyimides based on electron donor and acceptor substituted triphenylamines. Macromolecules 45, 3749 (2012).
T. Michinobu, C. Seo, K. Noguchi, and T. Mori: Effects of click postfunctionalization on thermal stability and field effect transistor performances of aromatic polyamines. Polym. Chem. 3, 1427 (2012).
C.-J. Chen, Y.-C. Hu, and G.-S. Liou: Linkage and acceptor effects on diverse memory behavior of triphenylamine-based aromatic polymers. Polym. Chem. 4, 4162 (2013).
O. Malinkiewicz, A. Yella, Y.H. Lee, G.M. Espallargas, M. Graetzel, M.K. Nazeeruddin, and H.J. Bolink: Perovskite solar cells employing organic charge-transport layers. Nat. Photon. 8, 128 (2014).
H.-J. Yen, J.-H. Lin, Y.O. Su, and G.-S. Liou: Novel triarylamine-based aromatic polyamides bearing secondary amines: synthesis and redox potential inversion characteristics induced by pyridines. J. Mater. Chem. C 4, 10381 (2016).
K. Suwa, S. Tanaka, K. Oyaizu, and H. Nishide: Arylamine polymers prepared via facile paraldehyde addition condensation: an effective hole-transporting materials for perovskite solar cells. Polym. Int. 67, 670 (2018).
L. Bian, E. Zhu, J. Tang, W. Tang, and F. Zhang: Recent progress in the design of narrow bandgap conjugated polymers for high-efficiency organic solar cells. Prog. Polym. Sci. 37, 1292 (2012).
C. Liu, K. Wang, X. Gong, and A.J. Heeger: Low bandgap semiconducting polymers for polymeric photovoltaics. Chem. Soc. Rev. 45, 4825 (2016).
Y. Wang and T. Michinobu: Benzothiadiazole and its π-extended, heteroannulated derivatives: useful acceptor building blocks for high-performance donor-acceptor polymers in organic electronics. J. Mater. Chem. C 4, 6200 (2016).
L. Ying, F. Huang, and G.C. Bazan: Regioregular narrow-bandgap-conjugated polymers for plastic electronics. Nat. Commun. 8, 14047 (2017).
Y. Li, M. Gu, Z. Pan, B. Zhang, X. Yang, J. Gu, and Y. Chen: Indacenodithiophene: a promising building block for high performance polymer solar cells. J. Mater. Chem. A 5, 10798 (2017).
P. Sonar, S.P. Singh, Y. Li, M.S. Soh, and A. Dodabalapur: A low-bandgap diketopyrrolopyrrole-benzothiadiazole-based copolymer for high-mobility ambipolar organic thin-film transistors. Adv. Mater. 22, 5409 (2010).
Z. Yi, S. Wang, and Y. Liu: Design of high-mobility diketopyrrolopyrrole-based π-conjugated copolymers for organic thin-film transistors. Adv. Mater. 27, 3589 (2015).
W. Li, K.H. Hendriks, M.M. Wienk, and R.A.J. Janssen: Diketopyrrolopyrrole polymers for organic solar cells. Acc. Chem. Res. 49, 78 (2016).
A. Facchetti, L. Vaccaro, and A. Marrochi: Semiconducting polymers prepared by direct arylation polycondensation. Angew. Chem. Int. Ed. 51, 3520 (2012).
L.G. Mercier and M. Leclerc: Direct (hetero)arylation: a new tool for polymer chemists. Acc. Chem. Res. 46, 1597 (2013).
S. Kowalski, S. Allard, K. Zilberberg, T. Riedl, and U. Scherf: Direct arylation polycondensation as simplified alternative for the synthesis of conjugated (co)polymers. Prog. Polym. Sci. 38, 1805 (2013).
A.E. Rudenko and B.C. Thompson: Optimization of direct arylation polymerization (DArP) through the identification and control of defects in polymer structure. J. Polym. Sci. Part A 53, 135 (2015).
J.-R. Pouliot, F. Grenier, J.T. Blaskovits, S. Beaupré, and M. Leclerc: Direct (hetero)arylation polymerization: simplicity for conjugated polymer synthesis. Chem. Rev. 116, 14225 (2016).
T. Bura, J.T. Blaskovits, and M. Leclerc: Direct (hetero)arylation polymerization: trends and perspectives. J. Am. Chem. Soc. 138, 10056 (2016).
S.-L. Suraru, J.A. Lee, and C.K. Luscombe: C-H arylation in the synthesis of π-conjugated polymers. ACS Macro Lett. 5, 724 (2016).
H. Bohra and M. Wang: Direct C-H arylation; a “greener” approach towards facile synthesis of organic semiconducting molecules and polymers. J. Mater. Chem. A 5, 11550 (2017).
S. Yu, F. Liu, J. Yu, S. Zhang, C. Cabanetos, Y. Gao, and W. Huang: Eco-friendly direct (hetero)-arylation polymerization: scope and limitation. J. Mater. Chem. C 5, 29 (2017).
P. Berrouard, A. Najari, A. Pron, D. Gendron, P.-O. Morin, J.-R. Pouliot, J. Veilleux, and M. Leclerc: Synthesis of 5-alkyl[3,4-c]thienopyrrole-4,6-dione-based polymers by direct heteroarylation. Angew. Chem. Int. Ed. 51, 2068 (2012).
S.-W. Chang, H. Waters, J. Kettle, Z.-R. Kuo, C.-H. Li, C.-Y. Yu, and M. Horie: Pd-catalysed direct arylation polymerisation for synthesis of low-bandgap conjugated polymers and photovoltaic performance. Macromol. Rapid Commun. 33, 1927 (2012).
Y. Gao, X. Zhang, H. Tian, J. Zhang, D. Yang, Y. Geng, and F. Wang: High mobility ambipolar diketopyrrolopyrrole-based conjugated polymer synthesized via direct arylation polycondensation. Adv. Mater. 27, 6753 (2015).
H. Song, Y. Deng, Y. Gao, Y. Jiang, H. Tian, D. Yan, Y. Gen, and F. Wang: Donor-acceptor conjugated polymers based on indacenodithiophene derivative bridged diketopyrrolopyrroles: synthesis and semiconducting properties. Macromolecules 50, 2344 (2017).
H. Saito, J. Chen, J. Kuwabara, T. Yasuda, and T. Kanbara: Facile one-pot access to π-conjugated polymers via sequential bromination/direct arylation polycondensation. Polym. Chem. 8, 3006 (2017).
Y. Li, W.J.K. Tatum, J.W. Onorato, S.D. Barajas, Y.Y. Yang, and C.K. Luscombe: An indacenodithiophene-based semiconducting polymer with high ductility for stretchable organic electronics. Polym. Chem. 8, 5185 (2017).
J. Kuwabara, T. Yasuda, S.J. Choi, W. Lu, K. Yamazaki, S. Kagaya, L. Han, and T. Kanbara: Direct arylation polycondensation: a promising method for the synthesis of highly pure, high-molecular-weight conjugated polymers needed for improving the performance of organic photovoltaics. Adv. Funct. Mater. 24, 3226 (2014).
W. Li and T. Michinobu: Structural effects of dibromocarbazoles on direct arylation polycondensation with 3,4-ethylenedioxythiophene. Polym. Chem. 7, 3165 (2016).
W. Li, M. Otsuka, T. Kato, Y. Wang, T. Mori, and T. Michinobu: 3,6-Carbazole vs 2,7-carbazole: a comparative study of hole-transporting polymeric materials for inorganic-organic hybrid perovskite solar cells. Beilstein J. Org. Chem. 12, 1401 (2016).
J.-R. Pouliot, B. Sun, M. Leduc, A. Najari, Y. Li, and M. Leclerc: A high mobility DPP-based polymer obtained via direct (hetero)arylation polymerization. Polym. Chem. 6, 278 (2015).
K. Wang, G. Wang, and M. Wang: Balanced ambipolar poly(diketopyrrolopyrrole-alt-tetrafluorobenzene) semiconducting polymers synthesized via direct arylation polymerization. Macromol. Rapid Commun. 36, 2162 (2015).
M.B. Gawande, S.N. Shelke, R. Zboril, and R.S. Varma: Microwave-assisted chemistry: synthetic applications for rapid assembly of nanomaterials and organics. Acc. Chem. Res. 47, 1338 (2014).
C.J. Mueller, E. Gann, C.R. Singh, M. Thelakkat, and C.R. McNeill: Role of the dopants on the morphological and transport properties of spiro-MeOTAD hole transport layer. Chem. Mater. 28, 7088 (2016).
Y. Wang, T. Hasegawa, H. Matsumoto, T. Mori, and T. Michinobu: Rational design of high-mobility semicrystalline conjugated polymers with tunable charge polarity: beyond benzobisthiadiazole-based polymers. Adv. Funct. Mater. 27, 1604608 (2017).
Y. Wang, T. Hasegawa, H. Matsumoto, T. Mori, and T. Michinobu: High-performance n-channel organic transistors using high-molecular-weight electron-deficient copolymers and amine-tailed self-assembled monolayers. Adv. Mater. 30, 1707164 (2018).
I. Lee, J.H. Yun, H.J. Son, and T.-S. Kim: Accelerated degradation due to weakened adhesion from Li-TFSi additives in perovskite solar cells. ACS Appl. Mater. Interfaces 9, 7029 (2017).
R. Schölin, M.H. Karlsson, S.K. Eriksson, H. Siegbahn, E.M.J. Johansson, and H. Rensmo: Energy level shifts in spiro-OMeTAD molecular thin films when adding Li-TFSI. J. Phys. Chem. C 116, 26300 (2012).
S.N. Habisreutinger, N.K. Noel, H.J. Snaith, and R.J. Nicholas: Investigating the role of 4-tert butylpyridine in perovskite solar cells. Adv. Energy Mater. 7, 1601079 (2017).
E.J. Juarez-Perez, M.R. Leyden, S. Wang, L.K. Ono, Z. Hawash, and Y. Qi: Role of the dopants on the morphological and transport properties of spiro-MeOTAD hole transport layer. Chem. Mater. 28, 5702 (2018).
Y. Xiao, G. Han, J. Wu, and J.-Y. Lin: Efficient bifacial perovskite solar cell based on a highly transparent poly(3,4-ethylenedioxythiophene) as the p-type hole-transporting material. J. Power Sources 306, 171 (2016).
A. Abate, T. Leijtens, S. Pathak, J. Teuscher, R. Avolio, M.E. Errico, J. Kirkpatrik, J.M. Ball, P. Docampo, I. McPherson, and H.J. Snaith: Lithium salts as “redox active” p-type dopants for organic semiconductors and their impact in solid-state dye-sensitized solar cells. Phys. Chem. Chem. Phys. 15, 2572 (2013).
ACKNOWLEDGMENTS
This work was supported by the Heiwa Nakajima Foundation, the Ogasawara Foundation for the Promotion of Science and Technology, the Yazaki Memorial Foundation for Science and Technology, the SEI Group CRS Foundation, and the Support for Tokyotech Advanced Researcher.
Author information
Authors and Affiliations
Corresponding author
Appendices
Supplementary materials
The supplementary material for this article can be found at https://doi.org/10.1557/mrc.2018.119.
Table of contents
Hole-transporting conjugated polymers synthesized by direct arylation polycondensation show higher hole mobilities and better photovoltaic performances of the perovskite solar cells than those synthesized by the conventional Stille coupling polycondensation.
Rights and permissions
About this article
Cite this article
Li, W., Mori, T. & Michinobu, T. Perovskite solar cells based on hole-transporting conjugated polymers by direct arylation polycondensation. MRS Communications 8, 1244–1253 (2018). https://doi.org/10.1557/mrc.2018.119
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/mrc.2018.119