Abstract
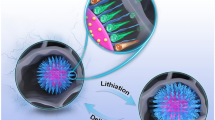
Graphene nanoribbons as a quasi-one-dimensional form of graphene has attracted intensive attention in energy related devices. Upon oxidation and cutting of multiwall carbon nanotubes (MWCNTs), highly dispersive graphene oxide nanoribbons (GONRs) were obtained, on which Zn2+ and Sn4+ can be homogenously deposited. The reduced graphene oxide nanoribbons (rGONRs)/Zn2SnO4 composite with a homogeneous distribution of nanoparticles on the nanoribbons have been prepared through facile in situ chemical co-reduction process. It is worth noting that the size of Zn2SnO4 particles tightly dispersed on rGONRs is about 15 nm. Benefit from the introduction of rGONRs, the specific surface area and electrode conductivity of rGONRs/Zn2SnO4 can both be effectively enhanced. The as-prepared rGONRs/Zn2SnO4 as anode material for lithium-ion batteries displays desirable electrochemical performance (727.2 mA h/g after 50 cycles at the current density of 100 mA/g), which is mainly attributed to the uniformly distributed Zn2SnO4 nanoparticles and the immobilizing and conducting effects of rGONRs.








Similar content being viewed by others
References
Q. Peng, Y. Li, X. He, X. Gui, Y. Shang, C. Wang, C. Wang, W. Zhao, S. Du, E. Shi, P. Li, D. Wu, and A. Cao: Graphene nanoribbon aerogels unzipped from carbon nanotube sponges. Adv. Mater. 26 (20), 3241 (2014).
X. Li, X. Wang, L. Zhang, S. Lee, and H. Dai: Chemically derived, ultrasmooth graphene nanoribbon semiconductors. Science 319 (5867), 1229 (2008).
L. Jiao, L. Zhang, X. Wang, G. Diankov, and H. Dai: Narrow graphene nanoribbons from carbon nanotubes. Nature 458 (7240), 877 (2009).
J. Lin, Z. Peng, C. Xiang, G. Ruan, Z. Yan, D. Natelson, and J.M. Tour: Graphene nanoribbon and nanostructured SnO2 composite anodes for lithium ion batteries. ACS Nano 7 (7), 6001 (2013).
O. Hod, V. Barone, J.E. Peralta, and G.E. Scuseria: Enhanced half-metallicity in edge-oxidized zigzag graphene nanoribbons. Nano Lett. 7 (8), 2295 (2007).
B. Luo, S. Liu, and L. Zhi: Chemical approaches toward graphene-based nanomaterials and their applications in energy-related areas. Small 8 (5), 630 (2012).
B. Genorio, W. Lu, A.M. Dimiev, Y. Zhu, A-R.O. Raji, B. Novosel, L.B. Alemany, and J.M. Tour: In situ intercalation replacement and selective functionalization of graphene nanoribbon stacks. ACS Nano 6 (5), 4231 (2012).
J. Lin, A-R.O. Raji, K. Nan, Z. Peng, Z. Yan, E.L.G. Samuel, D. Natelson, and J.M. Tour: Iron oxide nanoparticle and graphene nanoribbon composite as an anode material for high-performance Li-ion batteries. Adv. Funct. Mater. 24 (14), 2044 (2014).
Y. Yang, L. Li, H. Fei, Z. Peng, G. Ruan, and J.M. Tour: Graphene nanoribbon/V2O5 cathodes in lithium-ion batteries. ACS Appl. Mater. Interfaces 6 (12), 9590 (2014).
D.V. Kosynkin, A.L. Higginbotham, A. Sinitskii, J.R. Lomeda, A. Dimiev, B.K. Price, and J.M. Tour: Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 458 (7240), 872 (2009).
A.L. Higginbotham, D.V. Kosynkin, A. Sinitskii, Z. Sun, and J.M. Tour: Lower-defect graphene oxide nanoribbons from multiwalled carbon nanotubes. ACS Nano 4 (4), 2059 (2010).
T. Bhardwaj, A. Antic, B. Pavan, V. Barone, and B.D. Fahlman: Enhanced electrochemical lithium storage by graphene nanoribbons. J. Am. Chem. Soc. 132 (36), 12556 (2010).
Q. Qu, S. Yang, and X. Feng: 2D sandwich-like sheets of iron oxide grown on graphene as high energy anode material for supercapacitors. Adv. Mater. 23 (46), 5574 (2011).
W.S. Hummers and R.E. Offeman: Preparation of graphitic oxide. J. Am. Chem. Soc. 80 (6), 1339 (1958).
S. Chen, J. Zhu, X. Wu, Q. Han, and X. Wang: Graphene oxide−MnO2 nanocomposites for supercapacitors. ACS Nano 4 (5), 2822 (2010).
H. Wang, H.S. Casalongue, Y. Liang, and H. Dai: Ni(OH)2 nanoplates grown on graphene as advanced electrochemical pseudocapacitor materials. J. Am. Chem. Soc. 132 (21), 7472 (2010).
X.J. Zhu, L.M. Geng, F.Q. Zhang, Y.X. Liu, and L.B. Cheng: Synthesis and performance of Zn2SnO4 as anode materials for lithium ion batteries by hydrothermal method. J. Power Sources 189 (1), 828 (2009).
K. Kim, A. Annamalai, S.H. Park, T.H. Kwon, M.W. Pyeon, and M-J. Lee: Preparation and electrochemical properties of surface-charge-modified Zn2SnO4 nanoparticles as anodes for lithium-ion batteries. Electrochim. Acta 76, 192 (2012).
A. Rong, X.P. Gao, G.R. Li, T.Y. Yan, H.Y. Zhu, J.Q. Qu, and D.Y. Song: Hydrothermal synthesis of Zn2SnO4 as anode materials for Li-ion battery. J. Phys. Chem. B 110 (30), 14754 (2006).
Y. Zhao, Y. Huang, W. Zhang, Q. Wang, K. Wang, M. Zong, and X. Sun: Botryoidalis hollow Zn2SnO4 boxes@graphene as anode materials for advanced lithium-ion batteries. RSC Adv. 3 (45), 23489 (2013).
W. Song, J. Xie, W. Hu, S. Liu, G. Cao, T. Zhu, and X. Zhao: Facile synthesis of layered Zn2SnO4/graphene nanohybrid by a one-pot route and its application as high-performance anode for Li-ion batteries. J. Power Sources 229, 6 (2013).
W.S. Yuan, Y.W. Tian, and G.Q. Liu: Synthesis and electrochemical properties of pure phase Zn2SnO4 and composite Zn2SnO4/C. J. Alloys Compd. 506 (2), 683 (2010).
Y.R. Lim, C.S. Jung, H.S. Im, K. Park, J. Park, W.I. Cho, and E.H. Cha: Zn2GeO4 and Zn2SnO4 nanowires for high-capacity lithium- and sodium-ion batteries. J. Mater. Chem. A 4 (27), 10691 (2016).
K. Wang, Y. Huang, Y. Shen, L. Xue, H. Huang, H. Wu, and Y. Wang: Graphene supported Zn2SnO4 nanoflowers with superior electrochemical performance as lithium-ion battery anode. Ceram. Int. 40 (9), 15183 (2014).
H. Fan, Z. Liu, J. Yang, C. Wei, J. Zhang, L. Wu, and W. Zheng: Surfactant-free synthesis of Zn2SnO4 octahedron decorated with nanoplates and its application in rechargeable lithium ion batteries. RSC Adv. 4 (91), 49806 (2014).
Y.J. Hong and Y.C. Kang: Formation of core–shell-structured Zn2SnO4-carbon microspheres with superior electrochemical properties by one-pot spray pyrolysis. Nanoscale 7 (2), 701 (2015).
H.Y. Wang, B.Y. Wang, J.K. Meng, J.G. Wang, and Q.C. Jiang: One-step synthesis of Co-doped Zn2SnO4–graphene–carbon nanocomposites with improved lithium storage performances. J. Mater. Chem. A 3 (3), 1023 (2015).
Y. Zhao, Y. Huang, X. Sun, H. Huang, K. Wang, M. Zong, and Q. Wang: Hollow Zn2SnO4 boxes wrapped with flexible graphene as anode materials for lithium batteries. Electrochim. Acta 120, 128 (2014).
C. Yan, J. Yang, Q. Xie, Z. Lu, B. Liu, C. Xie, S. Wu, Y. Zhang, and Y. Guan: Novel nanoarchitectured Zn2SnO4 anchored on porous carbon as high performance anodes for lithium ion batteries. Mater. Lett. 138, 120 (2015).
H. Wang, Y. Wang, Z. Hu, and X. Wang: Cutting and unzipping multiwalled carbon nanotubes into curved graphene nanosheets and their enhanced supercapacitor performance. ACS Appl. Mater. Interfaces 4 (12), 6827 (2012).
H. Huang, Y. Huang, M. Wang, X. Chen, Y. Zhao, K. Wang, and H. Wu: Preparation of hollow Zn2SnO4 boxes@C/graphene ternary composites with a triple buffering structure and their electrochemical performance for lithium-ion batteries. Electrochim. Acta 147, 201 (2014).
E. Yoo, J. Kim, E. Hosono, H.S. Zhou, T. Kudo, and I. Honma: Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett. 8 (8), 2277 (2008).
S. Stankovich, D.A. Dikin, R.D. Piner, K.A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S.T. Nguyen, and R.S. Ruoff: Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45 (7), 1558 (2007).
C. Zhong, J. Wang, Z. Chen, and H. Liu: SnO2–graphene composite synthesized via an ultrafast and environmentally friendly microwave autoclave method and its use as a superior anode for lithium-ion batteries. J. Phys. Chem. C 115 (50), 25115 (2011).
C. Wang, H. Li, J. Zhao, Y. Zhu, W.Z. Yuan, and Y. Zhang: Graphene nanoribbons as a novel support material for high performance fuel cell electrocatalysts. Int. J. Hydrogen Energy 38 (30), 13230 (2013).
F. Han, W.C. Li, M.R. Li, and A.H. Lu: Fabrication of superior-performance SnO2@C composites for lithium-ion anodes using tubular mesoporous carbon with thin carbon walls and high pore volume. J. Mater. Chem. 22 (19), 9645 (2012).
B.Y. Wang, H.Y. Wang, Y.L. Ma, X.H. Zhao, W. Qi, and Q.C. Jiang: Facile synthesis of fine Zn2SnO4 nanoparticles/graphene composites with superior lithium storage performance. J. Power Sources 281, 341 (2015).
K. Wang, Y. Huang, T. Han, Y. Zhao, H. Huang, and L. Xue: Facile synthesis and performance of polypyrrole-coated hollow Zn2SnO4 boxes as anode materials for lithium-ion batteries. Ceram. Int. 40 (1), 2359 (2014).
Y. Zhao, Y. Huang, Q. Wang, K. Wang, M. Zong, L. Wang, W. Zhang, and X. Sun: Preparation of hollow Zn2SnO4 boxes for advanced lithium-ion batteries. RSC Adv. 3 (34), 14480 (2013).
S. Yuvaraj, W.J. Lee, C.W. Lee, and R.K. Selvan: In situ and ex situ carbon coated Zn2SnO4 nanoparticles as promising negative electrodes for Li-ion batteries. RSC Adv. 5 (82), 67210 (2015).
W. Song, J. Xie, S. Liu, G. Cao, T. Zhu, and X. Zhao: Graphene-induced confined crystal growth of octahedral Zn2SnO4 and its improved Li-storage properties. J. Mater. Res. 27 (24), 3096 (2012).
X. Ji, X. Huang, Q. Zhao, A. Wang, and X. Liu: Facile synthesis of carbon-coated Zn2SnO4 nanomaterials as anode materials for lithium-ion batteries. J. Mater. Res. 2014, 1 (2014).
A.K. Rai, J. Gim, L.T. Anh, and J. Kim: Partially reduced Co3O4/graphene nanocomposite as an anode material for secondary lithium ion battery. Electrochim. Acta 100, 63 (2013).
T. Jiang, X. Tian, H. Gu, H. Zhu, and Y. Zhou: Zn2SnO4@C core–shell nanorods with enhanced anodic performance for lithium-ion batteries. J. Alloys Compd. 639, 239 (2015).
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 51272075, No. 51372080 and No. 51238002). This work was also financially supported by the China Scholarship Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jing, M., Hou, Z., Yang, H. et al. Zn2SnO4 coated reduced graphene oxide nanoribbons with enhanced electrochemical performance for lithium-ion batteries. Journal of Materials Research 31, 3666–3674 (2016). https://doi.org/10.1557/jmr.2016.431
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2016.431