Abstract
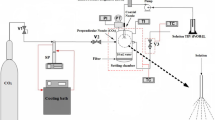
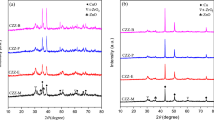
Three important low valent transition metal oxides were synthesized in supercritical methanol by using inorganic metal salts as precursors. X-ray powder diffraction, scanning electron microscopy, transmission electron microscopy, and x-ray photoelectron spectroscopy were applied to analyze the composition, structure, and morphology of the products. Results showed that Cu2O, MoO2, and V2O3 were obtained successfully under a supercritical condition of 240 °C and 9.0 MPa. MoO2 and V2O3 displayed sphere-like morphology with average particle sizes of 20–40 and 20–50 nm, respectively. Cu2O particles displayed edge-truncated cubic morphology with a particle size of 2.5 µm. Formation mechanism proposed that high valent metal oxides (CuO, MoO3, and V2O5) were formed firstly in supercritical methanol by the decomposing of precursors and then reduced to target products by free hydroxyl anions. In addition, methanol performed important roles not only as a reaction medium but also as a reducing agent under supercritical fluid conditions.





Similar content being viewed by others
References
C.H. Kuo and M.H. Huang: Morphologically controlled synthesis of Cu2O nanocrystals and their properties. Nano Today 5, 106–116 (2010).
H. Hu, J. Xu, C. Deng, and X. Ge: Easily controllable synthesis of alpha-MoO3 nanobelts and MoO2 microaxletrees through one-pot hydrothermal route. J. Nanosci. Nanotechnol. 14, 4462–4468 (2014).
A.C. Santulli, W. Xu, J.B. Parise, L. Wu, M.C. Aronson, F. Zhang, C.Y. Nam, C.T. Black, A.L. Tiano, and S.S. Wong: Synthesis and characterization of V2O3 nanorods. Phys. Chem. Chem. Phys. 11, 3718–3726 (2009).
Y. Zhao, W. Wang, Y. Li, Y. Zhang, Z. Yan, and Z. Huo: Hierarchical branched Cu2O nanowires with enhanced photocatalytic activity and stability for H2 production. Nanoscale 6, 195–198 (2014).
B. Koo, H. Xiong, M.D. Slater, V.B. Prakapenka, M. Balasubramanian, P. Podsiadlo, C.S. Johnson, T. Rajh, and E.V. Shevchenko: Hollow iron oxide nanoparticles for application in lithium ion batteries. Nano Lett. 12, 2429–2435 (2012).
L. Guan, H. Pang, J. Wang, Q. Lu, J. Yin, and F. Gao: Fabrication of novel comb-like Cu2O nanorod-based structures through an interface etching method and their application as ethanol sensors. Chem. Commun. 46, 7022–7024 (2010).
Y.H. Tsai, K. Chanda, Y.T. Chu, C.Y. Chiu, and M.H. Huang: Direct formation of small Cu2O nanocubes, octahedra, and octapods for efficient synthesis of triazoles. Nanoscale 6, 8704–8709 (2014).
L. Wu, P.O. Jubert, D. Berman, W. Imaino, A. Nelson, H. Zhu, S. Zhang, and S. Sun: Monolayer assembly of ferrimagnetic Co(x)Fe(3− x)O4 nanocubes for magnetic recording. Nano Lett. 14, 3395–3399 (2014).
Y. Xu, H. Wang, Y. Yu, L. Tian, W. Zhao, and B. Zhang: Cu2O Nanocrystals: Surfactant-free room-temperature morphology-modulated synthesis and shape-dependent heterogeneous organic catalytic activities. J. Phys. Chem. C 115, 15288–15296 (2011).
R. Srivastava, M.U. Anu Prathap, and R. Kore: Morphologically controlled synthesis of copper oxides and their catalytic applications in the synthesis of propargylamine and oxidative degradation of methylene blue. Colloids Surf., A 392, 271–282 (2011).
H-Y. Wu, M-H. Hon, C-Y. Kuan, and I-C. Leu: Preparation of TiO2 nanosheets by a hydrothermal process and their application as an anode for lithium-ion batteries. J. Electron. Mater. 43, 1048–1054 (2014).
D.S. Wang, T. Xie, Q. Peng, S.Y. Zhang, J. Chen, and Y.D. Li: Direct thermal decomposition of metal nitrates in octadecylamine to metal oxide nanocrystals. Chem.–Eur. J. 14, 2507–2513 (2008).
V.V. Lenka Matějová, R. Fajgar, Z. Matěj, V. Holý, and O. Šolcová: Reverse micelles directed synthesis of TiO2–CeO2 mixed oxides and investigation of their crystal structure and morphology. J. Solid State Chem. 198, 485–495 (2013).
M.D. Susman, Y. Feldman, A. Vaskevich, and I. Rubinstein: Chemical deposition of Cu2O nanocrystals with precise morphology control. ACS Nano 8, 162–174 (2014).
L.M. Sikhwivhilu, S.K. Pillai, and T.K. Hillie: Influence of citric acid on SnO2 nanoparticles synthesized by wet chemical processes. J. Nanosci. Nanotechnol. 11, 4988–4994 (2011).
D. Wang and Y. Li: Controllable synthesis of Cu-based nanocrystals in ODA solvent. Chem. Commun. 47, 3604–3606 (2011).
M. Cao, C. Hu, Y. Wang, Y. Guo, C. Guo, and E. Wang: A controllable synthetic route to Cu, Cu2O, and CuO nanotubes and nanorods. Chem. Commun. 15, (2003) 1884–1885.
X. Liang, L. Gao, S. Yang, and J. Sun: Facile synthesis and shape evolution of single-crystal cuprous oxide. Adv. Mater. 21, 2068–2071 (2009).
K. Giannousi, G. Sarafidis, S. Mourdikoudis, A. Pantazaki, and C. Dendrinou-Samara: Selective synthesis of Cu2O and Cu/Cu2O NPs: Antifungal activity to yeast saccharomyces cerevisiae and DNA interaction. Inorg. Chem. 53, 9657–9666 (2014).
C.V. Ramana, S. Utsunomiya, R.C. Ewing, and U. Becker: Formation of V2O3 nanocrystals by thermal reduction of V2O5 thin films. Solid State Commun. 137, 645–649 (2006).
C. Zheng, X. Zhang, S. He, Q. Fu, and D. Lei: Preparation and characterization of spherical V2O3 nanopowder. J. Solid State Chem. 170, 221–226 (2003).
F. Sediri and N. Gharbi: Hydrothermal synthesis and characterization of V2O3. Mater. Sci. Eng., B 123, 136–138 (2005).
K. Zhang, X. Sun, G. Lou, X. Liu, H. Li, and Z. Su: A new method for preparing V2O3 nanopowder. Mater. Lett. 59, 2729–2731 (2005).
Y. Shi, B. Guo, S.A. Corr, Q. Shi, Y.S. Hu, K.R. Heier, L. Chen, R. Seshadri, and G.D. Stucky: Ordered mesoporous metallic MoO2 materials with highly reversible lithium storage capacity. Nano Lett. 9, 4215–4220 (2009).
L.C. Yang, Q.S. Gao, Y. Tang, Y.P. Wu, and R. Holze: MoO2 synthesized by reduction of MoO3 with ethanol vapor as an anode material with good rate capability for the lithium ion battery. J. Power Sources 179, 357–360 (2008).
B. Veriansyah, J-D. Kim, B.K. Min, and J. Kim: Continuous synthesis of magnetite nanoparticles in supercritical methanol. Mater. Lett. 64, 2197–2200 (2010).
S. Desmoulins-Krawiec, C. Aymonier, and A. Loppinet-Serani, F.O. Weill, S.P. Gorsse, J. Etourneau, and F.O. Cansell: Synthesis of nanostructured materials in supercritical ammonia: Nitrides, metals and oxides. J. Mater. Chem. 14, 228 (2004).
N.C. Shin, Y-H. Lee, Y.H. Shin, J. Kim, and Y-W. Lee: Synthesis of cobalt nanoparticles in supercritical methanol. Mater. Chem. Phys. 124, 140–144 (2010).
F. Cansell and C. Aymonier: Design of functional nanostructured materials using supercritical fluids. J. Supercrit. Fluids 47, 508–516 (2009).
J-Y. Chang, J-J. Chang, B. Lo, S-H. Tzing, and Y-C. Ling: Silver nanoparticles spontaneous organize into nanowires and nanobanners in supercritical water. Chem. Phys. Lett. 379, 261–267 (2003).
K.J. Ziegler, R.C. Doty, K.P. Johnston, and B.A. Korgel: Synthesis of organic monolayer-stabilized copper nanocrystals in supercritical water. J. Am. Chem. Soc. 123, 7797–7803 (2001).
K. Sue, A. Suzuki, M. Suzuki, K. Arai, Y. Hakuta, H. Hayashi, and T. Hiaki: One-Pot synthesis of nickel particles in supercritical water. Ind. Eng. Chem. Res. 45, 623–626 (2005).
S. Marre, F. Cansell, and C. Aymonier: Design at the nanometre scale of multifunctional materials using supercritical fluid chemical deposition. Nanotechnology 17, 4594–4599 (2006).
C. Slostowski, S. Marre, O. Babot, T. Toupance, and C. Aymonier: Near- and supercritical alcohols as solvents and surface modifiers for the continuous synthesis of cerium oxide nanoparticles. Langmuir 28, 16656–16663 (2012).
O. Pascu, S. Marre, C. Aymonier, and A. Roig: Ultrafast and continuous synthesis of crystalline ferrite nanoparticles in supercritical ethanol. Nanoscale 5, 2126–2132 (2013).
J. Kim, D. Kim, B. Veriansyah, J. Won Kang, and J-D. Kim: Metal nanoparticle synthesis using supercritical alcohol. Mater. Lett. 63, 1880–1882 (2009).
H. Choi, B. Veriansyah, J. Kim, J-D. Kim, and J.W. Kang: Continuous synthesis of metal nanoparticles in supercritical methanol. J. Supercrit. Fluids 52, 285–291 (2010).
B. Veriansyah, M-S. Chun, and J. Kim: Surface-modified cerium oxide nanoparticles synthesized continuously in supercritical methanol: Study of dispersion stability in ethylene glycol medium. Chem. Eng. J. 168, 1346–1351 (2011).
A. Nugroho, S.J. Kim, W. Chang, K.Y. Chung, and J. Kim: Facile synthesis of hierarchical mesoporous Li4Ti5O12 microspheres in supercritical methanol. J. Power Sources 244, 164–169 (2013).
X. Liu, Y. Zhang, S. Yi, C. Huang, J. Liao, H. Li, D. Xiao, and H. Tao: Preparation of V2O3 nanopowders by supercritical fluid reduction. J. Supercrit. Fluids 56, 194–200 (2011).
S. Yu, S. Li, X. Ge, M. Niu, H. Zhang, C. Xu, and W. Li: Influence of reducing atmosphere of subcritical/supercritical mild alcohols on the synthesis of copper powder. Ind. Eng. Chem. Res. 53, 2238–2243 (2014).
S. Li, X. Ge, S. Jiang, X. Peng, Z. Zhang, W. Li, and S. Yu: Synthesis of octahedral and cubic Cu2O microcrystals in sub- and super-critical methanol and their photocatalytic performance. J. Mater. Sci. 50, 4115–4121 (2015).
V. Pessey, R. Garriga, F. Weill, B. Chevalier, J. Etourneau, and F. Cansell: Control of particle growth by chemical transformation in supercritical CO2/ethanol mixtures. J. Mater. Chem. 12, 958–965 (2002).
K.M.K. Selim, J-H. Lee, S-J. Kim, Z. Xing, I-K. Kang, Y. Chang, and H. Guo: Surface modification of magnetites using maltotrionic acid and folic acid for molecular imaging. Macromol. Res. 14, 646–653 (2006).
K.K. Selim, Z.C. Xing, M.J. Choi, Y. Chang, H. Guo, and I.K. Kang: Reduced cytotoxicity of insulin-immobilized CdS quantum dots using PEG as a spacer. Nanoscale Res. Lett. 6, 528 (2011).
J-M. Andanson, P.A. Bopp, and J-C. Soetens: Relation between hydrogen bonding and intramolecular motions in liquid and supercritical methanol. J. Mol. Liq. 129, 101–107 (2006).
D.T. Sawyer and J.L. Roberts: Hydroxide ion: An effective one-electron reducing agent? Acc. Chem. Res. 21, 469–476 (1988).
S.D. Gardner, C.S.K. Singamsetty, G.L. Booth, G-R. He, and C.U. Pittman: Surface characterization of carbon fibers using angle-resolved XPS and ISS. Carbon 33, 587–595 (1995).
K. Chen and D. Xue: Crystallisation of cuprous oxide. Int. J. Nanotechnol. 10, 4–12 (2013).
X. Wang, S. Jiao, D. Wu, Q. Li, J. Zhou, K. Jiang, and D. Xu: A facile strategy for crystal engineering of Cu2O polyhedrons with high-index facets. CrystEngComm 15, 1849 (2013).
G.H. Chan, J. Zhao, E.M. Hicks, G.C. Schatz, and R.P. Van Duyne: Plasmonic properties of copper nanoparticles fabricated by nanosphere lithography. Nano Lett. 7, 1947–1952 (2007).
B. Hu, L. Mai, W. Chen, and F. Yang: From MoO3 nanobelts to MoO2 nanorods: Structure transformation and electrical transport. ACS Nano 3, 478–482 (2009).
D. Weber, A. Stork, S. Nakhal, C. Wessel, C. Reimann, W. Hermes, A. Muller, T. Ressler, R. Pottgen, T. Bredow, R. Dronskowski, and M. Lerch: Bixbyite-type V2O3—a metastable polymorph of vanadium sesquioxide. Inorg. Chem. 50, 6762–6766 (2011).
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial supports by the Program for Liaoning Excellent Talents in University, China (Grant No. LR2012012) and (Grant No. LJQ2012034), and the Scientific Research Fund of Liaoning Provincial Education Department, China (Grant No. L2015423).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, S., Zhang, Z., Jiang, S. et al. Facile preparation and formation mechanism of three low valent transition metal oxides in supercritical methanol. Journal of Materials Research 31, 1440–1447 (2016). https://doi.org/10.1557/jmr.2016.149
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2016.149