Abstract
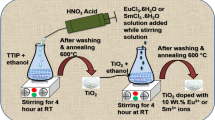
In this work, we report on the optoelectronic and photocatalytic features of europium (Eu3+)-doped TiO2 nanoscale particles synthesized via a sol-gel mediated rapid-condensation technique. X-ray diffraction studies have revealed the mixed phases of the synthesized systems. In particular, a mixture of anatase, brookite, and rutile phases was found to coexist beyond a sintering temperature of 600 °C while a pure anatase phase was witnessed below 500 °C. The photoluminescence spectra of ∼7 nm sized anatase TiO2 nanoparticles have exhibited different intra 4f (Eu3+ ion related) transitions with the most intense red emission (5D0→7F2) peak located at ∼613 nm. The emissions due to color centers and oxygen vacancies of TiO2 were also evident in the PL spectra. The Brunauer-Emmett-Teller surface area analysis has revealed a significant increment of surface area and pore volume owing to the enhanced interfacial region introduced by point defects and dislocations due to Eu doping. The photocatalytic activity of the Eu3+ doped TiO2 nanoscale system was found to be ∼12% stronger than its un-doped counterpart, as assessed from the degradation of methyl orange (MO) solution under UV light irradiation. The percentage of degradation was found to be strongly dependent on the duration of the UV exposure and Eu doping concentration. As an efficient photosensitive candidate, rare earth sensitized TiO2 systems would bring new insights while displaying both optoelectronic and photocatalytic characteristics through use of the localized states present in the band gap of the host.








Similar content being viewed by others
References
J.H. Braun, A. Baidins, and R.E. Marganski: TiO2 pigment technology: A review. Prog. Org. Coat. 20, 105 (1992).
G. Pfaff and P. Reynders: Angle-dependent optical effects deriving from submicron structures of films and pigments. Chem. Rev. 99, 1963 (1999).
S.A. Yuan, W.H. Chen, and S.S. Hu: Fabrication of TiO2 nanoparticles/surfactant polymer complex film on glassy carbon electrode and its application to sensing trace dopamine. Mater. Sci. Eng., C 25(4), 479 (2005).
C.F. Lin, C.H. Wu, and Z.N. Onn: Degradation of 4-chlorophenol in TiO2, WO3, SnO2, TiO2/WO3 and TiO2/SnO2 systems. J. Hazard. Mater. 154, 1033 (2008).
D. Chatterjee and A. Mahata: Demineralization of organic pollutants on the dye modified TiO2 semiconductor particulate system using visible light. Appl. Catal., B 33(2), 119 (2001).
H.G. Yu, S.C. Lee, J.G. Yu, and C.H. Ao: Photocatalytic activity of dispersed TiO2 particles deposited on glass fibers. J. Mol. Catal. A: Chem. 246, 206 (2006).
J. Yin, L. Xiang, and X. Zhao: Monodisperse spherical mesoporous Eu-doped TiO2 phosphor particles and the luminescence properties. Appl. Phys. Lett. 90, 113112 (2007).
A. Conde-Gallardo, M. Garcia-Rocha, I. Hernandez-Calderon, and R. Palomino-Merino: Photoluminescence properties of the Eu3+ activator ion in the TiO2 host matrix. Appl. Phys. Lett. 78, 3436 (2001).
J.G. Li, X. Wang, K. Watanabe, and T. Ishigaki: Phase structure and luminescence properties of Eu3+-doped TiO2 nanocrystals synthesized by Ar/O2 radio frequency thermal plasma oxidation of liquid precursor mists. J. Phys. Chem. B 110(3), 1121 (2006).
L.Y. Hu, H.W. Song, G.H. Pan, B. Yan, R.F. Qin, Q.L. Dai, L.B. Fan, S.W. Li, and X. Bai: Photoluminescence properties of samarium-doped TiO2 semiconductor nanocrystalline powders. J. Lumin. 127(2), 371 (2007).
K. Wilke and H.D. Breuer: The influence of transition metal doping on the physical and photocatalytic properties of titania. J. Photochem. Photobiol., A 121(1), 49 (1999).
K.L. Frindell, M.H. Bartl, A. Popitsch, and G.D. Stucky: Sensitized luminescence of trivalent europium by three-dimensionally arranged anatase nanocrystals in mesostructured titania thin films. Angew. Chem. Int. Ed. 114(6), 1001 (2002).
P. Yang, C. Lu, N. Hua, and Y. Du: Titanium dioxide nanoparticles co-doped with Fe3+ and Eu3+ ions for photocatalysis. Mater. Lett. 57, 794 (2002).
W. Xiaohong, Q. Wei, D. Xianbo, W. Yang, L. Huiling, and J. Zhaohua: Photocatalytic activity of Eu-doped TiO2 ceramic films prepared by microplasma oxidation method. J. Phys. Chem. Solids 68, 2387 (2007).
Q. Xiao, Z. Si, Z. Yu, and G. Qiu: Characterization and photocatalytic activity of Sm3+-doped TiO2 nanocrystalline prepared by low temperature combustion method. J. Alloys Compd. 450(1–2), 426 (2008).
K.T. Ranjit, I. Willner, S.H. Bossmann, and A.M. Braun: Lanthanide oxide-doped titanium dioxide photocatalysts: Novel photocatalysts for the enhanced degradation of p- chlorophenoxyacetic acid. Environ. Sci. Technol. 35, 1544 (2001).
D.C. Hurum, A.G. Agrios, K.A. Gray, T. Rajh, and M.C. Thurnauer: Explaining the enhanced photocatalytic activity of Degussa P25 mixed-phase TiO2 using EPR. J. Phys. Chem. B 107(19), 4545 (2003).
T. Ohno, K. Tokieda, S. Higashida, and M. Matsumura: Synergism between rutile and anatase TiO2 particles in photocatalytic oxidation of naphthalene. Appl. Catal., A 244(2), 383 (2003).
U. Diebold: The surface science of titanium dioxide. Surf. Sci. Rep. 48, 53 (2003).
Y. Liao, W. Que, Q. Jia, Y. He, J. Zhang, and P. Zhong: Controllable synthesis of brookite/anatase/rutile TiO2 nanocomposites and single-crystalline rutile nanorods array. J. Mater. Chem. 22(16), 7937 (2012).
B. Choudhury, B. Borah, and A. Choudhury: Extending photocatalytic activity of TiO2 nanoparticles to visible region of illumination by doping of cerium. Photochem Photobiol., 88(2), 257 (2012).
J. Jaćimović, C. Vâju, R. Gaál, A. Magrez, H. Berger, and L. Forró: High-pressure study of anatase TiO2. Materials 3(3), 1509 (2010).
D.R. Coronado, G.R. Gattorno, M.E. Espinosa-Pesqueira, C. Cab, R. de Coss, and G. Oskam: Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 19, 145605 (2008).
H. Zhang and J.F. Banfield: Phase transformation of nanocrystalline anatase-to-rutile via combined interface and surface nucleation. J. Mater. Res. 15(2), 437 (2000).
S.B. Qadri, E.F. Skelton, D. Hsu, A.D. Dinsmore, J. Yang, H.F. Gray, and B.R. Ratna: Size- induced transition-temperature reduction in nanoparticles of ZnS. Phys. Rev. B 60(13), 9191 (1999).
H. Zhang and J.F. Banfield: Understanding polymorphic phase transformation behavior during growth of nanocrystalline aggregates: Insights from TiO2. J. Phys. Chem. B 104, 3481 (2000).
S. Bakardjieva, V. Stengl, L. Szatmary, J. Subrt, J. Lukac, N. Murafa, D. Niznansky, K. Cizek, J. Jirkovsky, and N. Petrova: Transformation of brookite-type TiO2 nanocrystals to rutile: Correlation between microstructure and photoactivity. J. Mater. Chem. 16, 1709 (2006).
N. Paul, M. Patowary, B. Pegu, and D. Mohanta: Physical properties of nanoscale TiO2 related to Ag-doping and photochromic behavior. Nanosci. Nanotechnol. Lett. 5, 452 (2013).
M. Pal, U. Pal, J.M.G.Y. Jiménez, and F. Pérez-Rodríguez: Effects of crystallization and dopant concentration on the emission behavior of TiO2: Eu nanophosphors. Nanoscale Res. Lett. 7(1), 1 (2012).
Y.H. Tseng, C-S. Kuo, C-H. Huang, Y-Y. Li, P-W. Chou, C-L. Cheng, and M-S. Wong: Visible-light-responsive nano-TiO2 with mixed crystal lattice and its photocatalytic activity. Nanotechnology 17, 2490 (2006).
A.B. Murphy: Band-gap determination from diffuse reflectance measurements of semiconductor films and application to photoelectrochemical water-splitting. Sol. Energy Mater. Sol. Cells 91, 1326 (2007).
S.P. Singh and B. Karmakar: Photoluminescence enhancement of Eu3+ by energy transfer from Bi2+to Eu3+ in bismuth glass nanocomposites. RSC Advances 1, 751 (2011).
Y. Lei, L.D. Zhang, G.W. Meng, G.H. Li, X.Y. Zhang, C.H. Liang, W. Chen, and S.X. Wang: Preparation and photoluminescence of highly orderedTiO2 nanowire arrays. Appl. Phys. Lett. 78(8), 1125 (2001).
D. Li, H. Haneda, N.K. Labhsetwar, S. Hishita, and N. Ohashi: Visible-light-driven photocatalysis on fluorine-doped TiO2 powders by the creation of surface oxygen vacancies. Chem. Phys. Lett. 401, 579 (2005).
J.C. Yu, J. Yu, W. Ho, Z. Jiang, and L. Zhang: Effect of F-doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders. Chem. Mater. 14, 3808 (2002).
T. Tachikawa, T. Ishigaki, J-G. Li, M. Fujitsuka, and T. Majima: Defect-mediated photoluminescence dynamics of Eu3+ doped TiO2 nanocrystals revealed at the single- particle or single-aggregate level. Angew. Chem. Int. Ed. 47, 5348 (2008).
S. Das, A.A. Reddy, S. Ahmad, R. Nagarajan, and G.V. Prakash: Synthesis and optical characterization of strong red light emitting KLaF4: Eu3+nanophosphors. Chem. Phys. Lett. 508, 117 (2011).
Y. Zhang, F. Leung-Yuk Lam, Z.F. Yan, and X. Hu: Review of Kelvin’s equation and its modification in characterization of mesoporous materials. Chin. J. Chem. Phys. 19, 102 (2006).
N. Dutta, D. Mohanta, and A. Choudhury: Synthesis and pore filling mechanism in anatase TiO2 nanostructured network mediated by PbS molecular adsorption. J. Appl. Phys. 109, 094904 (2011).
A. Testino, I.R. Bellobono, V. Buscaglia, C. Canevali, M. D'Arienzo, S. Polizzi, R. Scotti, and F. Morazzoni: Optimizing the photocatalytic properties of hydrothermal TiO2 by the control of phase composition and particle morphology: A systematic approach. J. Am. Chem. Soc. 129(12), 3564 (2007).
A.J. Hoffman, E.R. Carraway, and M.R. Hoffman: Photocatalytic production of H2O2 and organic peroxides on quantum-sized semiconductor colloids. Environ. Sci. Technol. 28, 776 (1994).
F. Mahdavi, T.C. Burton, and Y. Li: Photoinduced reduction of nitro compounds on semiconductor particles. J. Org. Chem. 58(3), 744 (1993).
F.B. Li, X.Z. Li, M.F. Hou, K.W. Cheah, and W.C.H. Choy: Enhanced photocatalytic activity of Ce3+-TiO2 for 2-mercaptobenzothiazole degradation in aqueous suspension for odour control. Appl. Catal., A 285, 181 (2005).
Y-H. Xu, C. Chen, X-L. Yang, X. Li, and B-F. Wang: Preparation, characterization and photocatalytic activity of the neodymium-doped TiO2 nanotubes. Appl. Surf. Sci. 255, 8624 (2009).
Acknowledgments
One of the authors (NP) acknowledges DST, New Delhi for providing fellowship through INSPIRE scheme. We thank SAIF, NEHU, Shillong for extending TEM facility. NP would like to thank Ms. Giti Das for her assistance during synthesis steps. The authors also thank Prof. R.C. Deka, Dr. G.A. Ahmed, and Ms. Rasna Devi for their help and support in BET surface analysis through N2 adsorption/desorption study. A part of the work was carried out through project no. UFR-50307/2011 supported by IUAC, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paul, N., Mohanta, D. Effective optoelectronic and photocatalytic response of Eu3+-doped TiO2 nanoscale systems synthesized via a rapid condensation technique. Journal of Materials Research 28, 1471–1480 (2013). https://doi.org/10.1557/jmr.2013.122
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2013.122