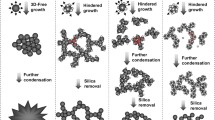
Abstract
A strategic scheme for controlling the shape of titania nanorods while maintaining their highly crystallized state was investigated in terms of the effects of reactant concentration and temperature change on the formation mechanism. Lowering the temperature from 433 to 413 K markedly slowed down the reaction rate and resulted in the coexistence of amorphous-like films and crystalline titania nanorods due to the concurrence of nucleation out of the amorphous phase and particle growth by crystallization. Based on these findings, a strategy for shape control was proposed and long, high aspect ratio titania nanorods in a highly crystallized state were successfully synthesized.













Similar content being viewed by others
References
G. Centi and S. Perathoner: The role of nanostructure in improving the performance of electrodes for energy storage and conversion. Eur. J. Inorg. Chem. 2009, 3851 (2009).
X. Hu, G. Li, and J.C. Yu: Design, fabrication, and modification of nanostructured semiconductor materials for environmental and energy applications. Langmuir 26, 3031 (2010).
L. Manna, E.C. Scher, and A.P. Alivisatos: Synthesis of soluble and processable rod-, arrow-, teardrop-, and tetrapod-shaped CdSe nanocrystals. J. Am. Chem. Soc. 122, 12700 (2000).
S.A. Empedocles, R. Neuhauser, K. Shimizu, and M.G. Bawendi: Photoluminescence from single semiconductor nanostructures. Adv. Mater. 11, 1243 (1999).
M. Nirmal and L. Brus: Luminescence photophysics in semiconductor nanocrystals. Acc. Chem. Res. 32, 407 (1999).
A. Kongkanand and P.V. Kamat: Electron storage in single wall carbon nanotubes. Fermi level equilibration in semiconductor–SWCNT suspensions. ACS Nano 1, 13 (2007).
M. Law, L.E. Green, J.C. Johnson, R. Saykally, and P. Yang: Nanowire dye-sensitized solar cells. Nat. Mater. 4, 455 (2005).
A.P. Alivisatos: Semiconductor clusters, nanocrystals, and quantum dots. Science 271, 933 (1996).
A.B.F. Martinson, M.S. Goes, F. Febregat-Santiago, J. Bisquert, M.J. Pellin, and J.T. Hupp: Electron transport in dye-sensitized solar cells based on ZnO Nanotubes: Evidence for highly efficient charge collection and exceptionally rapid dynamics. J. Phys. Chem. A 113, 4015 (2009).
C. Burda, X. Chen, R. Narayanan, and El-M.A. Sayed: Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 105, 1025 (2005).
C.B. Murray, C.R. Kagan, and M.G. Bawendi: Synthesis and characterization of monodisperse nanocrystals and close-packed nanocryatal assemblies. Annu. Rev. Mater. Sci. 30, 545 (2000).
E.C. Scher, R. Soc, L. Manna, and A.P. Alivisatos: Shape control and applications of nanocrystals. Philos. Trans. R. Soc. London, Ser. A 361, 241 (2003).
X.G. Peng, L. Manna, W.D. Yang, J. Wickham, E. Scher, A. Kadavanich, and A.P. Alivisatos: Shape control of CdSe nanocrystals. Nature 404, 59 (2000).
Q. Song and Z.J. Zhang: Shape control and associated magnetic properties of spinel cobalt ferrite nanocrystals. J. Am. Chem. Soc. 126, 6164 (2004).
B.D. Reiss, C. Mao, D.J. Solis, K.S. Ryan, T. Thomson, and A.M. Belcher: Biological routes to metal alloy ferromagnetic nanostructures. Nano Lett. 4, 1127 (2004).
W W. Yu, Y.A. Wang, and X. Peng: Formation and stability of size-, shape-, and structure-controlled CdTe nanocrystals: Ligand effects on monomers and nanocrystals. Chem. Mater. 15, 4300 (2003).
Z. Tang, B. Ozturk, Y. Wang, and N.A. Kotov: Simple preparation strategy and one-dimensional energy transfer in CdTe nanoparticle chains. J. Phys. Chem. B 108, 6927 (2004).
Z. Tang, N.A. Kotov, and M. Giersig: Spontaneous organization of single CdTe nanoparticles into luminescent nanowires. Science 297, 237 (2002).
H. Masuda and K. Fukuda: Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. Science 268, 1466 (1995).
H. Masuda, H. Yamada, M. Satoh, H. Asoh, M. Nakao, and T. Tamamura: Highly ordered nanochannel-array architecture in anodic alumina. Appl. Phys. Lett. 71, 2770 (1997).
K. Shankar, J.I. Basham, N.K. Allam, O.K. Varghese, G.K. Mor, X. Feng, M. Paulose, J.A. Seabold, K.-S. Choi, and C.A. Grimes: Recent advances in the use of TiO2 nanotube and nanowire arrays for oxidative photoelectrochemistry. J. Phys. Chem. C 113, 6327 (2009).
A. Ghicov and P. Schmuki: Self-ordering electrochemistry: A review on growth and functionality of TiO2 nanotubes and other self-aligned MOx structures. Chem. Commun. 2791 (2009).
S. Rani, S.C. Roy, M. Paulose, O.K. Varghese, G.K. Mor, S. Kim, S. Yoriya, T.J. Latempa, and C.A. Grimes: Synthesis and applications of electrochemically self-assembled titania nanotube arrays. Phys. Chem. Chem. Phys. 12, 2780 (2010).
C.-J. Lin, C.W. Yu-Y., and S.-H. Chien: Transparent electrodes of ordered opened-end TiO2-nanotube arrays for highly efficient dye-sensitized solar cells. J. Mater. Chem. 20, 1073 (2010).
R.L. Penn and J.F. Banfield: Morphology development and crystal growth in nanocrystalline aggregates under hydrothermal conditions: Insights from titania. Science 281, 969 (1998).
J.F. Banfield, S.A. Welch, H. Zhang, T.T. Ebert, and R.L. Penn: Aggregation-based crystal growth and microstructure development in natural iron oxyhydroxide biomineralization products. Science 289, 751 (2000).
K. Fujihara, A. Kumar, R. Jose, S. Ramakrishna, and S. Uchida: Spray deposition of electrospun TiO2 nanorods for dye-sensitized solar cell. Nanotechnology 18, 365709 (2007).
R.A. Lucky, Y. Medina-Gonzalez, and P.A. Charpentier: Zr doping on one-dimensional titania nanomaterials synthesized in supercritical carbon dioxide. Langmuir 26, 19014 (2010).
H.G. Yang, C.H. Sun, S.Z. Qiao, J. Zou, G. Liu, S.C. Smith, H.M. Cheng, and G.Q. Lu: Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 453, 638 (2008).
H.G. Yang, G. Liu, S.Z. Qiao, C.H. Sun, Y.G. Jin, S.C. Smith, J. Zou, H.M. Cheng, and G.Q. Lu: Solvothermal synthesis and photoreactivity of anatase TiO2 nanosheets with dominant {001} facets. J. Am. Chem. Soc. 131, 4078 (2009).
R. Garcia and R. Tello: Size and shape controlled growth of molecular nanostructures on silicon oxide templates. Nano Lett. 4, 1115 (2004).
M. Grätzel: Photoelectrochemical cells. Nature 414, 338 (2001).
M. Grätzel: Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 44, 6841 (2005).
M.K. Nazeeruddin, De F. Angelis, S. Fantacci, A. Selloni, G. Viscardi, P. Liska, S. Ito, T. Bessho, and M. Graetzel: Combined experimental and DFT-TDDFT computational study of photoelectrochemical cell ruthenium sensitizers. J. Am. Chem. Soc. 127, 16835 (2005).
T. Sawatsuk, A. Chindaduang, C. Sae-kung, S. Pratontep, and G. Tumcharern: Dye-sensitized solar cells based on TiO2–MWCNTs composite electrodes: Performance improvement and their mechanisms. Diamond Relat. Mater. 18, 524 (2009).
N. Cai, S.-J. Moon, L. Cevey-Ha, T. Moehl, R. Humphry-Baker, P. Wang, S.M. Zakeeruddin, and M. Graetzel: An organic D-pi-A dye for record efficiency solid-state sensitized heterojunction solar cells. Nano Lett. 11, 1452 (2011).
J.-H. Yum, E. Baranoff, S. Wenger, M.K. Nazeeruddin, and M. Graetzel: Panchromatic engineering for dye-sensitized solar cells. Energy Environ. Sci. 4, 842 (2011).
T. Bessho, S.M. Zakeeruddin, C.-Y. Yeh, E.W. Diau-G., and M. Grätzel: Highly efficient mesoscopic dye-sensitized solar cells based on donor-acceptor-substituted porphyrins. Angew. Chem. Int. Ed. 49, 6646 (2010).
A. Fujishima and K. Honda: Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37 (1972).
A. Kudo and Y. Miseki: Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 38, 253 (2009).
M. Adachi, Y. Murata, J. Takao, J. Jiu, M. Sakamoto, and F. Wang: Highly efficient dye-sensitized solar cells with titania thin film electrode composed of network structure of single-crystal-like TiO2 nanowires made by “oriented attachment” mechanism. J. Am. Chem. Soc. 126, 14943 (2004).
J. Jiu, S. Isoda, F. Wang, and M. Adachi: Dye-sensitized solar cells based on a single-crystalline TiO2 nanorod film. J. Phys. Chem. B 110, 2087 (2006).
T. Kurata, Y. Mori, S. Isoda, J. Jiu, K. Tsuchiya, F. Uchida, and M. Adachi: Characterization and formation process of highly crystallized single crystalline TiO2 nanorods for dye-sensitized solar cells. Curr. Nanosci. 6, 269 (2010).
M. Adachi, J. Jiu, and S. Isoda: Synthesis of morphology-controlled titania nanocrystals and application for dye-sensitized solar cells. Curr. Nanosci. 3, 285 (2007).
M. Adachi, J. Jiu, S. Isoda, Y. Mori, and F. Uchida: Self-assembled nanoscale architecture of TiO2 and application for dye-sensitized solar cells. Nanotechnol. Sci. Appl. 1, 1 (2008).
K. Yoshida, J. Jiu, D. Nagamatsu, T. Nemoto, H. Kurata, M. Adachi, and S. Isoda: Structure of TiO2 nanorods formed with double surfactants. Mol. Cryst. Liq. Cryst. 491, 14 (2008).
T. Sugimoto, X. Zhou, and A. Muramatsu: Synthesis of uniform anatase TiO2 nanoparticles by gel–sol method 4. Shape control. J. Colloid Interface Sci. 259, 53 (2003).
P.A. Connor, K.D. Dobson, and A.J. McQuillan: New sol-gel attenuated total reflection infrared spectroscopic method for analysis of adsorption at metal oxide surface in aqueous solution. Chelation of TiO2, ZrO2, and Al2O3 surfaces by catechol, 8-quinolinol, and acetylacetone. Langmuir 11, 4193 (1995).
J. Jiu, F. Wang, M. Sakamoto, J. Takao, and M. Adachi: Preparation of nanocrystaline TiO2 with mixed template and its application for dye-sensitized solar cells. J. Electrochem. Soc. 151, A1653 (2004).
J. Jiu, S. Isoda, M. Adachi, and F. Wang: Preparation of TiO2 nanocrystalline with 3–5 nm and application for dye-sensitized solar cell. J. Photochem. Photobiol., A 189, 314 (2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Motonari, A., Yoshida, K., Kurata, T. et al. Shape control of highly crystallized titania nanorods based on formation mechanism. Journal of Materials Research 27, 440–447 (2012). https://doi.org/10.1557/jmr.2011.393
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2011.393