Abstract
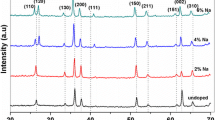
We present a low-temperature, hydrothermal synthesis method for Ta-doped TiO2. Here, alkoxide-based precursors are mixed at low temperatures to suppress differential hydrolysis and phase separation. This method ensures homogeneous, molecular mixing of the Ta dopant with the native oxide up to a concentration of ~2.5 at.%. X-ray diffraction and energy dispersive spectrometer analyses confirm a uniformly doped rutile TiO2. Scanning electron microscopy and transmission electron microscopy analyses reveal a highly branched structure. Optoelectronic properties of these structures were investigated using ultraviolet-visible spectroscopy and low-temperature photoluminescence.







Similar content being viewed by others
References
A.S. Barnard and P. Zapol: Effects of particle morphology and surface hydrogenation on the phase stability of TiO2. Phys. Rev. B 70 (23), 235403 (2004).
H.M. Cheng, J.M. Ma, Z.G. Zhao, and L.M. Qi: Hydrothermal preparation of uniform nanosize rutile and anatase particles. Chem. Mater. 7 (4), 663 (1995).
U. Diebold: The surface science of titanium dioxide. Surf. Sci. Rep. 48 (5–8), 53 (2003).
J. Du, X.Y. Lai, N.L. Yang, J. Zhai, D. Kisailus, F.B. Su, D. Wang, and L. Jiang: Hierarchically ordered macro-mesoporous TiO2-graphene composite films: Improved mass transfer, reduced charge recombination, and their enhanced photocatalytic activities. ACS Nano 5, 590 (2011).
A. Fujishima and K. Honda: Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37 (1972).
M.K. Nowotny and D.W. Bahnemann: Improved photocatalytic performance of rutile TiO2. Phys. Status Solidi RRL 5 (3), 92 (2011).
M. Gratzel: Photoelectrochemical cells. Nature 414, 338 (2001).
T. Ohno, M. Akiyoshi, T. Umebayashi, K. Asai, T. Mitsui, and M. Matsumura: Preparation of S-doped TiO2 photocatalysts and their photocatalytic activities under visible light. Appl. Catal., A 265, 115 (2004).
W. Zhou, Q. Liu, Z. Zhu, and J. Zhang: Preparation and properties of vanadium-doped TiO2 photocatalysts. J. Phys D: Appl. Phys. 43, 1 (2010).
X.C. Liu, F. Gao, L.L. Zhao, and C.S. Tian: Phase transition of low-temperature sintering tungsten-doped ZnO-TiO2 ceramics. J. Mater. Sci. Mater. Electron. 18, 863 (2007).
N. Couselo, F.S.G. Einschlag, R.J. Candal, and M. Jobbagy: Tungstun doped TiO2 vs pure TiO2 photocatalysts: Effects on photobleaching kinetics and mechanism. J. Phys. Chem. C 112, 1094 (2008).
R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki, and Y. Taga: Visible-light photocatalysis in nitrogen-doped titanium oxide. Science 293, 269 (2001).
S. Zhang, Z. Zhao, C. Liu, W. Dong, X. Zhang, and W. Chen: Study on the optical properties of Mn-doped TiO2 thin films. J. Mater. Sci. 39, 2909 (2004).
K.Y.S. Chan and G.K.L. Goh: Hydrothermal growth of ferromagnetic Fe-doped TiO2 films. Thin Solid Films 516, 5582 (2008).
G.K.L. Goh, K.Y.S. Chan, and T. Liu: Hydrothermal epitaxy of ferromagnetic cobalt doped titanium dioxide films at 120 °C. Cryst. Eng. Comm. 13, 524 (2011).
C. Wang, A. Geng, Y. Guo, S. Jiang, and X. Qu: Three-dimensionally ordered macroporous Ti1-xTaxO2+x/2 (x = 0.025, 0.05, and 0.075) nanoparticles: Preparation and enhanced photocatalytic activity. Mater. Lett. 60, 2711 (2006).
B.J. Morgan, D.O. Scanlon, and G.W. Watson: Small polarons in Nb- and Ta-doped rutile and anatase TiO2. J. Mater. Chem. 19, 5175 (2009).
S.C. Navale, A. Vadivel Murugan, and V. Ravi: Varistors based on Ta-doped TiO2. Ceram. Int. 33, 301 (2007).
C.C. Wang and J.Y. Ying: Sol-gel synthesis and hydrothermal processing of anatase and rutile titania nanocrystals. Chem. Mater. 11, 3113 (1999).
R. Long and N.J. English: Band gap engineering of (N, Ta)-codoped TiO2: A first-principles calculation. Chem. Phys. Lett. 478, 175 (2009).
X. Chen and S.S. Mao: Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 107, 2891 (2007).
N.M. Kinsinger, A. Wong, D. Li, F. Villalobos, and D. Kisailus: Nucleation and crystal growth of nanocrystalline anatase and rutile phase TiO2 from a water soluble precursor. Cryst. Growth Des. 10, 5254 (2010).
B.P. Jiang, H.B. Yin, T.S. Jiang, Y.H. Jiang, H. Feng, K.M. Chen, W.P. Zhou, and Y.J. Wada: Hydrothermal synthesis of rutile TiO2 nanoparticles using hydroxyl and carboxyl group-containing organics as modifiers. Mater. Chem. Phys. 98, 231 (2006).
X. Bokhimi, A. Morales, M. Aguilar, J.A. Toledo-Antonio, and F. Pedraza: Local order in titania polymorphs. Int. J. Hydrogen Energy 26, 1279 (2001).
S.D. Mo and W.Y. Ching: Electronic and optical properties of three phases of titanium dioxide: Rutile, anatase and brookite. Phys. Rev. B 51, 13023 (1995).
J.K. Burdett, T. Hughbanks, G.J. Miller, J.W. Richardson, and J.V. Smith: Structural electronic relationships in inorganic solids: Powder neutron diffraction studies of the rutile and anatase polymorphs of titanium-dioxide at 15 and 295 K. J. Am. Chem. Soc. 109, 3639 (1987).
Y. Yang, D.J. Miller, and S.B. Hawthorne: Toluene solubility in water and organic partitioning from gasoline and diesel fuel into water at elevated temperatures and pressures. J. Chem. Eng. Data 42, 908 (1997).
J. Livage and C. Sanchez: Sol-gel chemistry. J. Non-Cryst. Solids 145, 11 (1992).
Remington: The Science and Practice of Pharmacy (Philadelphia: Lippincott Williams & Wilkins, 2005).
R.P. Netterfield, P.J. Martin, C.G. Pacey, W.G. Sainty, D.R. Mckenzie, and G. Auchterlonie: Ion-assisted deposition of mixed TiO2–SiO2 films. J. Appl. Phys. 66, 1805 (1989).
J.L.G. Fierro, L.A. Arrua, J.M.L. Nieto, and G. Kremenic: Surface properties of Co-precipitated V–Ti–O catalysts and their relation to the selective oxidation of isobutene. Appl. Catal. 37, 323 (1988).
T. Siemens Meyer and J.W. Schultze: XPS and UPS studies of gas-phase oxidation, electrochemistry and corrosion behavior of Ti and Ti5Ta. Surf. Interface Anal. 16, 309 (1990).
S.F. Ho, S. Contarini, and J.W. Rabalais: Ion-beam induced chemical changes in oxyanions (Moyn) and oxides (Max) where M = chromium, molybdenum, tungsten, vanadium, niobium and tantalum. J. Phys. Chem. 91, 4779 (1987).
L. Granasy, T. Pusztai, G. Tegze, J.A. Warren, and J.F. Douglas: Growth and form of spherulites. Phys. Rev. E: Stat. Nonlinear Soft Matter Phys. 72, 011605 (2005).
H.D. Keith and F.J. Padden: A phenomenological theory of spherulitic crystallization. J. Appl. Phys. 34, 2409 (1963).
L. Shen, N. Bao, Y. Zheng, A. Gupta, T. An, and K. Yanagisawa: Hydrothermal splitting of titanate fibers to single-crystalline TiO2 nanostructures with controllable crystalline phase, morphology, microstructure, and photocatalytic activity. J. Phys. Chem. C 112, 8809 (2008).
C. Sanchez, J. Livage, M. Henry, and F. Babonneau: Chemical modification of alkoxide precursors. J. Non-Cryst. Solids 100, 65 (1988).
B. Ma, G.K.L. Goh, J. Ma, and T.J. White: Growth kinetics and cracking of liquid-phase-deposited anatase films. J. Electrochem. Soc. 154 (10), 557 (2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arab, S., Li, D., Zaera, F. et al. Solvothermal synthesis of a highly branched Ta-doped TiO2. Journal of Materials Research 26, 2653–2659 (2011). https://doi.org/10.1557/jmr.2011.286
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2011.286