Abstract
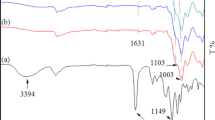
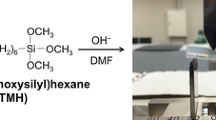
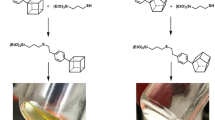
The introduction of organic substituents into sol-gel materials can often result in networks that collapse during drying to afford non-porous xerogels. This can prove useful if non-porous coatings or membranes are the ultimate objectives. Collapse of porosity is also manifested in bridged polysilsesquioxanes with flexible bridging groups. Alkylene-bridged polysilsesquioxanes are hybrid xerogels whose organic bridging group is an integral constituent of the network polymer that can be systematically varied to probe the influence of its length on the xerogels’ porosity and morphology. Our previous studies have shown that hexylene-bridged polysilsesquioxane xerogels prepared from 1,6-bis(triethoxysilyl)hexane under acidic conditions are nonporous while the pentylene-bridged polysilsesquioxanes prepared under the same conditions are porous. We also discovered that the more reactive 1,6-bis(trimethoxysilyl)hexane monomer could polymerize under acidic conditions to afford porous xerogels. Here, we have extended our study of bis(trimethoxysilyl)alkanes to include the heptylene (C7), octylene (C8), nonylene(C9) and decylene (C10) bridges so as to ascertain at what bridging group length the porosity collapses. The morphology of the resulting xerogels was characterized by nitrogen sorption porosimetry and electron microscopy. Solid state NMR was used to structurally characterize the materials.
Similar content being viewed by others
References
K. J. Shea, J. J. E. Moreau, D. A. Loy, R. J. P. Corriu, B. Boury, “Functional Hybrids Based on Bridged Silsesquioxanes” Hybrid Functional Materials, ed. P. Gomez-Romero and C. Sanchez (Wiley, 2004) pp. 50–85.
K. J. Shea, D. A. Loy, Chem. Mater. 13(10), 3306 (2001).
D. A. Loy, K. J. Shea, Chem. Rev. 95(5), 1431 (1995).
H. W. Oviatt, K. J. Shea, J. H. Small, Chem. Mater. 5, 943 (1993).
D. A. Loy, G. M. Jamison, B. M. Baugher, E. M. Russick, R. A. Assink, S. Prabakar, K. J. Shea, J. Non-Cryst. Solids 186, 44 (1995).
D. A. Loy, B. M. Baugher, S. Prabakar, R. A. Assink, K. J. Shea in “Advances in Porous Materials” ed by S. Komarneni, D. M. Smith, and J. S. Beck (Mater. Res. Soc. Symp. Proc. 371, Pittsburg, PA, 1995) p 229.
Acknowledgments
Los Alamos National Laboratory, an affirmative action/equal opportunity employer, is operated by the University of California for the United States Department of Energy under contract W- 7405-ENG-36. Sandia is a multiprogram laboratory operated by Sandia Corporation, a Lockheed Martin Company, for the United States Department of Energy under Contract DE- AC04-94AL85000.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Loy, D.A., Small, J.H., DeFriend, K.A. et al. Collapse of Porosity During Drying of Alkylene-Bridged Polysilsesquioxane Gels. Influence of the Bridging Group Length. MRS Online Proceedings Library 847, 531–536 (2004). https://doi.org/10.1557/PROC-847-EE9.9
Published:
Issue Date:
DOI: https://doi.org/10.1557/PROC-847-EE9.9