Abstract
Bentonite may be used, in conjunction with cementitious materials, in repository designs for TRU wastes in Japan. Therefore, possible effects of the interaction of highly alkaline water with bentonite are of interest. In this study, the sorption of plutonium and uranium has been measured on a bentonite sample that had been altered by hydrothermal exposure to a high pH solution.
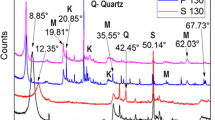
Samples of Kunipia-F® bentonite were exposed to a pH 14 solution of sodium, potassium and calcium hydroxides for 20 days at 130°C, 160°C and 200°C under air. X-Ray analysis showed that alteration at 160°C was similar to alteration at 200°C.
Batch sorption experiments were carried out at room temperature under nitrogen by contacting 200°C-altered bentonite with synthetic equilibrated water containing both uranium and plutonium in the presence of sodium dithionite as a reducing agent. The experiments were equilibrated for three months and then sampled after centrifugation, 0.45 gm filtration, and 10,000 nominal molecular weight cut-off (NMWCO) filtration. Uranium and plutonium remaining in solution were determined. The final redox potentials were between -500 to -51OmV vs SHE and the final pH values were 12.2.
Sorption of both actinides showed some dependence on the solid:liquid separation method. The average RD value for uranium sorption after centrifugation was 2 × 103cm3g−1 which increased to 3 × 104 cm3g−1 after 0.45 ώm filtration. However, the effect of a further decrease in the pore size of the filter had little additional effect; 10,000 NMWCO filtration gave an average RD of 5 × 104 cm3g−1. The sorption of plutonium after solid:liquid separation by centrifugation was very similar to uranium with an average RD of 6 × 103 cm3g−1. After 0.45 ώm filtration the increase in RD to 2 × 105 cm3g−1 was more marked than for uranium. There was no significant increase in RD value after 10,000 NMWCO filtration compared to 0.45 μm filtration.
Similar content being viewed by others
References
S. Ichige, M. Ito and M. Mihara, 1998 Annual Meeting of the Atomic Energy Society of Japan, 1998, p. 611 (in Japanese).
Japan Nuclear Cycle Development Institute, The Draft Second Progress Report on Research and Development for the Geological Disposal of HLW in Japan, 1999, (http://www.jnc.go.jp/kaihatu/tisou/zh12/Draft2nd/s02/pdf/04-01-02-01-02.pdf)
B.F. Greenfield, D.J. Ilett, M. Ito, R. McCrohon, T.G. Heath, C.J. Tweed, S.J. Williams and M. Yui, Radiochimica Acta 82, 27 (1998).
D. Rai, Radiochimica Acta 35, 97 (1984).
G.M.N. Baston, J.A. Berry, M. Brownsword, T.G. Heath, D.J. Ilett, C.J. Tweed and M. Yui in Scientific Basis for Nuclear Waste Management XX, edited by W.J. Gray and I.R. Triay MRS, Pittsburgh, PA, 1997) pp. 805–812.
Power Reactor and Nuclear Fuel Development Corporation, Annual Report of the Study on the Geological Disposal of HLW, 1996 (in Japanese).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brownsword, M., Mihara, M. & Williams, S.J. Sorption of Uranium and Plutonium on Bentonite Altered by Highly Alkaline Water. MRS Online Proceedings Library 608, 249 (1999). https://doi.org/10.1557/PROC-608-249
Published:
DOI: https://doi.org/10.1557/PROC-608-249