Abstract
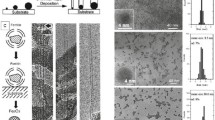
Several oxidants were examined for their abilities in opening carbon nanotube end caps. Up to 91% of carbon nanotubes were found to have at least one open end when treated by 0.2 M KMnCV 10% H2SO4 or 0.2 M KMnO4/0.2 M CrO3 aqueous solution at 100° C, 90 min. That is, -82% of carbon nanotubes was converted to “nanostraws”. The morphologies of the processed carbon nanotubes reflect the relative strength of these oxidants. The mechanism of carbon nanotube end cap opening processes will be discussed.
Similar content being viewed by others
References
S. Iijima, Nature 354, 56 (1991).
T. W. Ebbesen and P. M. Ajayan, Nature 358, 220 (1992).
J. W. Mintmire, B. I. Dunlap, C. T. White, Phys. Rev. Lett. 68, 631 (1992)
N. Hamada, S. I. Sawada, A. Oskiyama, ibid. 68, 1579 (1992)
R. Saito, M. Fujita, G. Dresselhaus, M. S. dresselhaus, Appl. Phys. Lett 60, 2204 (1992).
P. Ball, Nature 361, 297 (1993).
P. M. Ajayan and S. Iijima, Nature 361, 333 (1993).
S. C. Tsang, P. J. F. Harris and M. L. H. Green, Nature 362, 520 (1993).
P. M. Ajayan, T. W. Ebbesen, T. Ichiharhi, S. Iijima, K. Tanigaki and H. Hirua, Nature 362, 522 (1993).
S. Iijima, Nature 354, 56 (1991)
S. Iijima, T. Ichihashi, Y. Ando, ibid, 356, 776 (1992).
W. A. Waters, Q. Rev. Chem. Soc 12, 277 (1958).
B. Sklarz, Q. Rev. Chem. Soc. 21, 3 (1967)
H. Vorbrueggen and C. Djerassi, J. Am. Chem. Soc. 84, 2990 (1962)
F. D. Gunstone and P. J. Sykes, J. Chem. Soc. 1962, 3058.
J. R. Henry and S. M. Weinreb, J. Org. Chem. 58, 4745 (1993)
M. Uskokovic, M. Gut, E. N. Trachtenderg, W. Klyne and R. I. Dorfman, J. Am. Chem. Soc. 82, 4965 (1960)
M. Schroder, Chem. Rev. 80, 187 (1980).
G. Ohloff and W. Giersch, Angew. Chem. Int. Ed. Engl. 12, 401 (1973).
D. F. Tavres and J. P. Borger, Canad. J. Chem. 44, 1323 (1966)
J. Rocek and F. H. Westheimer, J. Am. Chem. Soc. 84, 2241 (1962).
K. C. Hwang, J. Chem. Soc. Chem. Comm. (in press).
E. Dujardin, T. W. Ebbesen, H. Hiura and K. Tanigaki, Science 265, 1850 (1994).
R. C. Haddon Ace. Chem. Soc. 21, 243 (1988); Science 261, 1545 (1993).
J. M. Hawkins, A. Meyer, M. A. Solow, J. Am. Chem. Soc. 115, 7499 (1993)
J. M. Hawkins, M. Nambu, A. Meyer, ibid. 116, 7642 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hwang, K.C. High Yield Conversion of Carbon Nanotubes to Nanostraws at Mild Conditions. MRS Online Proceedings Library 359, 75–80 (1994). https://doi.org/10.1557/PROC-359-75
Published:
Issue Date:
DOI: https://doi.org/10.1557/PROC-359-75