Abstract
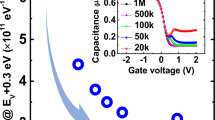
We analyze a main scheme for the suppression of GeO desorption by the high pressure oxidation which drastically improve the electrical quality of Ge/GeO2 capacitors. The inherent driving force for GeO to form at the Ge/GeO2 interface and to diffuse toward the GeO2 surface was realized by the concentration gradient in the GeO2 film, which was obtained from the thermodynamic calculation. Kinetic consideration based on the comparison with Si/SiO2 stacks suggests that GeO desorption at the GeO2 surface is the rate-limiting process under passive oxidation conditions. When O2 pressure is increased by high pressure oxidation, the vapor pressure of GeO at the GeO2 surface is reduced, restricting GeO desorption at the GeO2 surface.
Similar content being viewed by others
References
J. T. Law, and P. S. Meigs, J. Electrochem. Soc. 104, 154 (1957).
R. E. Schlier, and H. E. Farnsworth, J. Chem. Phys. 30, 917 (1959).
C. O. Chui, S. Ramanathan, B. B. Triplett, P. C. McIntyre, and K. C. Saraswat, Tech. Dig. IEDM, 437 (2002).
A. Ritenour , S. Yu, M. L. Lee, N. Lu, W. Bai, A. Pitera, E. A. Fitzgerald, D.-L. Kwong, and D. A. Antoniadis, Tech. Dig. IEDM, 433 (2004).
W. P. Bai, N. Lu, and D.-L. Kwong, IEEE EDL 26, 378 (2005).
Y. Kamata, Materials Today 11, 30 (2008).
C.H. Lee, T. Nishimura, K. Nagashio, K. Kita, and A. Toriumi, ECS Trans.(in press).
C. Wagner, J. Appl. Phys. 29, 1295 (1958).
E. A. Gulbransen and S. A. Jansson, Oxidation of Metals 4, 181 (1972).
F. W. Smith and G. Ghidini, J. Electrochem. Soc. 129, 1300 (1982).
A. Ishizuka and Y. Shiraki, J. Electrochem. Soc. 133, 666 (1986).
B. E. Deal and A. S. Grove, J. Appl. Phys. 36, 3770 (1965).
I. Barin, Thermodynamical Data of Pure Substances, PartI&II, VCH Verlags Gesellschaft, Weinheim, 1993.
N. Birks and G. H. Meier, Introduction to high temperature oxidation of metals, Edward Arnold, 1983, p. 42.
M. K. Schurman and M. Tomozawa, J. Non-Crystal. Soids 202, 93 (1996).
J. D. Kalen, R. S. Boyce, and J. D. Cawley, J. Am. Ceram. Soc. 74, 203 (1991).
K. Kita, S. Suzuki, H. Nomura, T. Takahashi, T. Nishimura, and A. Toriumi, Jpn. K. Appl. Phys. 47, 2349 (2008).
K. A. Jackson, Kinetic Processes, Wiley-VCH, 2004, p.97.
K. Kita, C. H. Lee, T. Nishimura, K. Nagashio, and A. Toriumi, ECS Trans. 16, 187 (2008).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nagashio, K., Lee, C.H., Nishimura, T. et al. Thermodynamics and Kinetics for Suppression of GeO Desorption by High Pressure Oxidation of Ge. MRS Online Proceedings Library 1155, 602 (2008). https://doi.org/10.1557/PROC-1155-C06-02
Received:
Accepted:
Published:
DOI: https://doi.org/10.1557/PROC-1155-C06-02