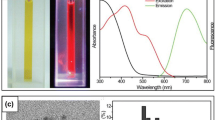
Abstract
Fluorescent metal nanoclusters, which consist of collections of small numbers of noble metal atoms, are of great interest in photochemistry and photophysics due to their strong size-dependent emission. Historically their generation was confined to gaseous and solid phases; however, recently a unique organic/inorganic hybrid materials approach was developed that utilizes dendrimers as templates to protect nanoclusters from solution based fluorescence quenching. These hybrid dendrimer/gold nanoclusters are water-soluble and highly fluorescent. Yet there are several intrinsic deficiencies in their synthetic method: first, NaBH4, a toxic chemical, was used as reducing agent in the reaction; and second, the reaction yield was low due to the concurrent formation of large, non-emissive, gold particles. Here we report a particle-free method to produce dendrimer-encapsulated gold nanoclusters in high-yield. Proof of concept is demonstrated using OH-terminated poly(amidoamine) dendrimer and Au(PX3)3Cl (X = Ph, Me), but the approach can also be extended to the combination of other dendrimers and organic noble metal salts. Our approach yields fluorescent clusters with homogeneous size distribution. These clusters can be transferred to aqueous solution and used directly for biological applications.
Similar content being viewed by others
References
P. Alivisatos, Nat. Biotechnol. 22 (1), 47 (2004); M. Bruchez, M. Moronne, P. Gin et al., Science 281 (5385), 2013 (1998); P. V. Kamat, J. Phys. Chem. B 106 (32), 7729 (2002); G. D. Liu, T. M. H. Lee, and J. S. Wang, J. Am. Chem. Soc. 127 (1), 38 (2005); C. A. Mirkin, Inorg. Chem. 39 (11), 2258 (2000).
A. P. Alivisatos, W. W. Gu, and C. Larabell, Annu. Rev. Biomed. Eng. 7, 55 (2005); J. K. Jaiswal and S. M. Simon, Trends Cell Biol. 14 (9), 497 (2004).
M. A. El-Sayed, Acc. Chem. Res. 34 (4), 257 (2001); S. Empedocles and M. Bawendi, Acc. Chem. Res. 32 (5), 389 (1999).
G. A. Ozin and S. A. Mitchell, Angew. Chem. Int. Ed. 22 (9), 674 (1983); R. M. Crooks, M. Zhao, L. Sun et al., Acc. Chem. Res. 34 (3), 181 (2001); Jun Li, Xi Li, Hua-Jin Zhai et al., Science 299 (5608), 864 (2003); J. G. Zhang, S. Q. Xu, and E. Kumacheva, Adv. Mater. 17 (19), 2336 (2005).
R. C. Jin, S. Egusa, and N. F. Scherer, J. Am. Chem. Soc. 126 (32), 9900 (2004); Y. Negishi and T. Tsukuda, Chem. Phys. Lett. 383 (1-2), 161 (2004); J. P. Wilcoxon, J. E. Martin, F. Parsapour et al., J. Chem. Phys. 108 (21), 9137 (1998); S. Link, A. Beeby, S. Fitzgerald et al., J. Phys. Chem. B 106 (13), 3410 (2002); Y. Negishi, Y. Takasugi, S. Sato et al., J. Am. Chem. Soc. 126 (21), 6518 (2004); M. F. Bertino, Z. M. Sun, R. Zhang et al., J. Phys. Chem. B 110 (43), 21416 (2006).
J. Zheng, J. T. Petty, and R. M. Dickson, J. Am. Chem. Soc. 125 (26), 7780 (2003).
J. Zheng, C. W. Zhang, and R. M. Dickson, Phys. Rev. Lett. 93 (7), 077402 (2004).
R. C. Triulzi, M. Micic, S. Giordani et al., Chem. Commun. (48), 5068 (2006).
Y. Bao, C. Zhong, D. Vu et al., J. Phys. Chem. C, submitted (2007).
M. L. Tran, A. V. Zvyagin, and T. Plakhotnik, Chem. Commun. (22), 2400 (2006).
D. J. Wang and T. Imae, J. Am. Chem. Soc. 126 (41), 13204 (2004).
M. Q. Zhao, L. Sun, and R. M. Crooks, J. Am. Chem. Soc. 120 (19), 4877 (1998).
Y. Shichibu, Y. Negishi, T. Tsukuda et al., J. Am. Chem. Soc. 127 (39), 13464 (2005).
M. Quinn and G. Mills, J. Phys. Chem. 98 (39), 9840 (1994); K. Esumi, T. Hosoya, A. Suzuki et al., Langmuir 16 (6), 2978 (2000).
D. Schonauer, H. Lauer, and U. Kreibig, Zeitschrift fur Physik D (Atoms, Molecules and Clusters) 20 (1/4), 301 (1991).
W. M. Pankau, K. Verbist, and G. von Kiedrowski, Chem. Commun. (6), 519 (2001).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhong, C., Bao, Y., Vu, D.M. et al. Fabrication of Fluorescent Cellular Probes: Hybrid Dendrimer/Gold Nanoclusters. MRS Online Proceedings Library 1007, 1507 (2007). https://doi.org/10.1557/PROC-1007-S15-07
Published:
DOI: https://doi.org/10.1557/PROC-1007-S15-07