Abstract
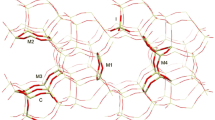
A combination of quantum-chemical approaches including DFT, semiempirical PM3 and molecular mechanics (force field MM+) methods has been applied for analysis of the structure of polynuclear hydroxocomplexes of Zr in diluted solutions of its oxochloride as precursors of zirconia nanoparticles in zirconia-pillared clays. Relative stability of complexes differing by their size and shape has been estimated.
Similar content being viewed by others
References
V.A. Sadykov, T.G.Kuznetsova. et al, Chemistry for Sustain. Developm. 11, 249 (2003).
T.G. Kuznetsova, V.A. Sadykov et al, RF Patent No. 2194573 of 17.09.01.
V. A. Sadykov, T.G. Kuznetsova et al. Mat. Res. Symp. Proc. 703, 529 (2002).
V.A. Sadykov et al, Topics in Catalysis 32, 29 (2005).
V.A. Sadykov, R.V. Bunina, et al, J. Catal. 2001, 200, 117.
B. G. Linsen, Academic P. Kess “Physical and Chemical aspects of adsorbents and catalysts”.London and New York, 1970, 332–383.
Inorganic crystal structure database (ICSD). Canad. Journ. Chem. 46, 3491 (1968).
Allinger, N.L. J. Am. Chem. Soc. 99:8127, 1977.
M. J. Frisch, G. W. Trucks, H. B. Schlegel at al., Gaussian 98, Revision A.11, Gaussian, Inc., Pittsburgh PA, 2001.
R.G. Parr, W. Yang, Density-Functional Theory of Atoms and Molecules, Oxford University Press, New York, 1989.
P. Hohenberg, W. Kohn, Phys.Rev. B 136 (1964) 864.
W. Kohn, L.S.Sham, Phys.Rev. A 140 (1965) 1133.
A.D. Becke, Phys. Rev. A, 38 (1988) 3098.
A.D. Becke, J. Chem. Phys. 98 (1993) 5648.
C. Lee, W. Yang, R.G. Parr, Phys. Rev. B, 37 (1988) 785.
R. Krishnan, J. S. Seger, J. A. Pople, J. Chem. Phys. 72 (1980) 650.
W. Stevens, H.Bash, J. Krauss, J. Chem. Phys. 81 (1984) 6026.
Clearfild, A. Rev. Pure & Appl. Chem. 1964, 14, 19.
J. Miehe-Brendle, L. Khouchaf, J. Baron, R. Le Dred, M.-H. Tuilier. Microporous Materials 11 (1997) 171–183.
Clearfild, A. J. Mater.Res. 1990, 5, 161.
Singhal, L.M. Toth, J.S.Lin, and K. Affholter J. Am. Chem. Soc. 1996, 118, 11529–11534.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mezentseva, N.V., Sadykov, V.A., Avdeev, V.I. et al. Analysis of the structural specificity of ZrO2 nanoparticles in pillared clays by modeling the condensation process in ZrOCl2*8H2O solutions. MRS Online Proceedings Library 894, 08940605 (2005). https://doi.org/10.1557/PROC-0894-LL06-05
Received:
Accepted:
Published:
DOI: https://doi.org/10.1557/PROC-0894-LL06-05