Abstract
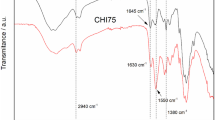
Starch suspension proves to be a useful matrix for the hydrolytic route to metal oxide nanoparticles, due to its size-stabilization effect, which works also at high temperatures. To understand the type of interaction between the organic part and the oxide particles, various parameters, such as viscosity, temperature, degree of polymerization, and organic/inorganic kinds of dispersant, are tested through x-ray diffraction (XRD), transmission electron microscopy (TEM), solid-state nuclear magnetic resonance (NMR), and thermogravimetric mass spectra (TG–MS) analyses of the obtained SnO2 nanopowders. Results highlight the unique role of starch compared with other hydrophilic dispersants that do not ensure effective size stabilization on curing up to 600 °C. The proof comes from the study of pyrolysis of the residual organic groups surrounding the particles. They are chelating carboxylic species that prevent the coalescence among metal oxide nanoparticles.













Similar content being viewed by others
References
X.G. Peng, L. Manna, W.D. Yang, J. Wickham, E. Scher, A. Kadavanich, and A.P. Alivisatos: Shape control of CdSe nanocrystals. Nature 404, 59 (2000).
Y. Sun and Y. Xia: Shape-controlled synthesis of gold and silver nanoparticles. Science 298, 2176 (2002).
X. Peng: Mechanisms for the shape-control and shape-evolution of colloidal semiconductor nanocrystals. Adv. Mater. 15(5), 459 (2003).
J. Liu, Y.L. Lam, Y.C. Chan, Y. Zhou, B.S. Ooi, and Z.S. Yun: Experimental and theoretical study of the cracking behavior of sol-gel-derived SiO2 film on InP substrate. J. Appl. Phys. A: Mater. Sci. Process. 70(3), 341 (2000).
X-C. Yuan, W.X. Yu, N.Q. Ngo, and W.C. Cheong: Improved sol-gel thin film for fabrication of multilevel structures using a high-energy beam-sensitive gray-scale mask. Opt. Eng. 42(2), 302 (2003).
W.X. Yu and X-C. Yuan: Fabrication of refractive microlens in hybrid SiO2/TiO2 sol-gel glass by electron-beam lithography. Opt. Express 11(8), 899 (2003).
C. Sakurai, T. Fukui, and M. Okuyama: Preparation of zirconia coatings by hydrolysis of zirconium alkoxide with hydrogen peroxide. J. Am. Ceram. Soc. 76(4), 1061 (1993).
H. Kozuka and M. Hirano: Radiative striations and surface roughness of alkoxide-derived spin coating films. J. Sol.-Gel Sci. Technol. 19(1), 501 (2000).
Y. Ohya, H. Saiki, and Y. Takahashi: Preparation of transparent, electrically conducting ZnO film from zinc acetate and alkoxide. J. Mater. Sci. 29(15), 4099 (1994).
Q. Ji and T. Shimizu: Chemical synthesis of transition metal oxide nanotubes in water using an iced lipid nanotube as a template. Chem. Commun. 4411 (2005).
T. Miyao, T. Saika, Y. Saito, and S. Naito: Preparation of alumina and silica–alumina nanotubes encapsulating platinum ultrafine particles. J. Mater. Sci. Lett. 22(7), 543 (2003).
S. Dirè, E. Pagani, F. Babonneau, R. Ceccato, and G. Carturan: Unsupported SiO2-based organic–inorganic membrane. Part 1. Synthesis and structural characterization. J. Mater. Chem. 7(1), 67 (1997).
C.J. Brinker and G.W. Scherer: Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing. (Academic Press, San Diego, CA, 1990).
C.J. Brinker, A.J. Hurd, P-R. Schunk, and C.S. Ashley: Review of sol-gel thin film formation. J. Non-Cryst. Solids 147 & 148, 424 (1992).
S. Ramesh, E. Sominska, B. Cina, R. Chaim, and A. Gedanken: Nanocrystalline _-alumina synthesized by sonohydrolysis of alkoxide precursor in the presence of organic acids: Structure and morphological properties. J. Am. Ceram. Soc. 83(1), 89 (2000).
D. Li and R.B. Kaner: Shape and aggregation control of nanoparticles: Not shaken, not stirred. J. Am. Chem. Soc. 128, 968 (2006).
Y. Xia, P. Yang, Y. Sun, Y. Wu, B. Mayers, B. Gates, Y. Yin, F. Kim, and H. Yan: One-dimensional nanostructures: Synthesis, characterization, and applications. Adv. Mater. 15(5), 353 (2003).
R.J. Hunter: Foundation of Colloidal Science. 1 (Oxford University Press, New York, 1987).
E. Callone, G. Carturan, and A. Sicurelli: Nanopowders of metallic oxides by the hydrolytic route with starch stabilization and biological abetment. J. Nanosci. Nanotech. 6(1), 254 (2006).
E. Callone, G. Carturan, M. Ischia, and A. Sicurelli: Nanometric oxides from molecular precursors in the presence of starch. Coatings of glass with these oxides in silica sols. J. Mater. Res. 21(7), 1726 (2006).
C. Canevali, N. Chiodini, F. Morazzoni, J. Padovani, A. Paleari, R. Scotti, and G. Spinolo: Substitutional tin doped silica glasses: An infrared study of the sol-gel transition. J. Non-Cryst. Solids 293–295, 32 (2001).
M. Acciarri, C. Canevali, C.M. Mari, M. Mattoni, R. Ruffo, R. Scotti, F. Morazzoni, D. Barreca, L. Armelao, E. Tondello, E. Bontempi, and L.E. Depero: Nanocrystalline SnO2-based thin films obtained by sol-gel route: A morphological and structural investigation. Chem. Mater. 15, 2646 (2003).
R. Dal Maschio, S. Dirè, G. Carturan, S. Enzo, and L. Battezzati: Phase separation in gel-derived materials, separation and crystallization of SnO2 within an amorphous SiO2 matrix. J. Mater. Res. 7(2), 435 (1992).
L. Lutterotti, S. Matthies, and H.R. Wenk: Proc. 12th International Conference on Textures of Materials (ICOTOM-12), 1999, p. 1599.
R. Campostrini, G.D. Sorarù, R. Ceccato, G. Carturan, and G. Dandrea: Pyrolysis study of methyl-substituted Si–H containing gels as precursors for oxycarbide glasses, by combined thermogravimetry, gas chromatographic and mass spectrometric analysis. J. Mater. Chem. 6, 585 (1996).
M.A. Rao, M.J. Cooley, and A.A. Vitali: Flow properties of concentrated juices at low temperatures. Food Technol. 38, 113 (1984).
J.Y. Thebaudin, A.C. Lefebvre, and J.L. Doublier: Rheology of starch pastes from starches of different origins: Application to starchbased sauces. Lebensm.-Wiss.U. Technol. 31(4), 354 (1998).
D.P. Tunstall, S. Patou, R.S. Liu, and Y.H. Kao: Size effects in the NMR of SnO2 powders. Mater. Res. Bull. 34, 1513 (1999).
D.C. Dragunski and A. Pawlicka: Preparation and characterization of starch grafted with toluene and polypropylene oxide diisocyanate. Mater. Res. 4(2), 77 (2001).
M.P. Williamson, C. Trevitt, and J.M. Noble: NMR studies of dextran oligomer interactions with model polyphenols. Carbohydr. Res. 266(2), 229 (1995).
R.E. Dinnebier, S. Vensky, M. Jansen, and J.C. Hanson: Crystal structures and topological aspects of the high-temperature phases and decomposition products of the alkali metal oxalates M2[C2O4] (M = K, Rb,Cs). Chem. Eur.J. 11, 1119 (2005).
M.A. Mohamed, A.K. Galwey, and S.A. Halawy: A comparative study of the thermal reactivities of some transition metal oxides in selected atmospheres. Thermochim. Acta 429, 57 (2005).
R.L. Frost and M.L. Weier: Thermal decomposition of humboldtine— a high resolution thermogravimetric and hot stage Raman spectroscopic study. J. Therm. Anal. Calorim. 75, 277 (2004).
E.J. Hehre: The biological synthesis of dextran from dextrins. J. Biol. Chem. 192, 162 (1951).
M.A. Larrubia Vargas, G. Busca, T. Montanari, M.C. Herrera Delgado, and L.J. Alemany: Preparation and characterization of silicon hydride oxide: A fully hydrophobic solid. J. Mater. Chem. 15, 910 (2005).
N. Chiodini, F. Morazzoni, A. Paleari, R. Scotti, and G. Spinolo: Sol-gel synthesis of monolithic tin-doped silica glass. J. Mater. Chem. 9, 2653 (1999).
Acknowledgments
We thank both Ministero per l’Universita e la Ricerca Scientifica e Tecnologica-Progetti Nazionali di Ricerca (MURST-PNR) 2001-2003 (FIRB art. 8) and “Provincia Autonoma di Trento” for funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Callone, E., Carturan, G., Ischia, M. et al. Size stabilization of nanoparticles by polysaccharides: Effectiveness in the wet and curing steps. Journal of Materials Research 22, 3344–3354 (2007). https://doi.org/10.1557/JMR.2007.0416
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/JMR.2007.0416