Abstract
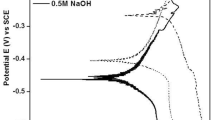
The corrosion behavior of the bulk glass-forming Mg65Y10Cu15Ag10 alloy was studied in neutral and weakly acidic media. Potentiodynamic polarization studies in cyclic and linear modes were carried out in electrolytes with a pH = 7, containing different anions. The alloy corroded freely in electrolytes with sulfate and pthalate ions, whereas passivity was observed in the electrolyte with borate ions. Further tests were performed in boric-acid-added borate buffer solution with pH = 7, 6, and 5. From Tafel characteristics, corrosion potentials and corrosion current densities were estimated. The data were compared with those of the ternary Mg65Y10Cu15 metallic glass. Potentiostatic anodic polarization tests were conducted on the Mg65Y10Cu15Ag10 alloy in boric-acid-added borate buffer solution with pH = 7 at two different potentials, 800 and 300 mV, saturated calomel electrode, which revealed different current transient characteristics. Auger electron spectroscopy was employed to characterize the anodically generated passive layers. The depth distributions of the elements as well as their chemical states were detected to be different for layers formed in electrolytes (i) with different pH values (8.4 and 7) of the same anion, (ii) with the same pH value but containing different anions (borate, sulfate, and pthalate), and (iii) with the same pH value and anion (borate) but at two different anodic potentials.
Similar content being viewed by others
References
B.L. Mordike and T. Ebert, Mater. Sci. Eng. A 302, 37 (2001).
R.F. Decker, Adv. Mater. Proc. 9, 31 (1998).
G.L. Song and A. Atrens, Adv. Eng. Mater. 1, 11 (1999).
A. Inoue, T. Zhang, and T. Matsumoto, Mater. Trans., JIM 31, 177 (1990).
A. Peker and W.L. Johnson, Appl. Phys. Lett. 63, 2342 (1993).
A. Inoue and T. Matsumoto, Mater. Sci. Eng. A 173, 1 (1993).
H.G. Kang, E.S. Park, W.T. Kim, and H.K. Cho, Mater. Trans., JIM 41, 846 (2000).
A. Gebert, K. Buchholz, A. Leonhard, K. Mummert, J. Eckert, and L. Schultz, Mater. Sci. Eng. A 267, 294 (1999).
V. Schroeder, C.J. Gilbert, and R.O. Ritchie, Scripta Mater. 38, 1481 (1998).
S. Hiromoto, A-P. Tsai, M. Sumita, and T. Hanawa, Corros. Sci. 42, 2193 (2000).
A. Gebert, U. Wolff, A. John, and J. Eckert, Scripta Mater. 43, 279 (2000).
A. Gebert, U. Wolff, A. John, J. Eckert, and L. Schultz, Mater. Sci. Eng. A 299, 125 (2001).
R.V. Subba Rao, U. Wolff, S. Baunack, J. Eckert, and A. Gebert, Corros. Sci. 45, 817 (2002).
H.H. Uhlig and J.R. Gilman, Z. Phys. Chem. 226, 127 (1964).
Y. Zuo, H. Wang, J. Zhao, and J. Xiong, Corros. Sci. 44, 13 (2002).
G.M. Florianovich, Y.M. Ko¨lotyrkin, D. Kononova, in Proceedings of the 4th ICMC-Amsterdam, edited by N.E. Hamner (NACE, Amsterdam, The Netherlands, 1972), p. 189.
S. Virtanen, P. Schmuki, M. Bu¨chler, H. Isaacs, J. Electrochem. Soc. 146, 4087 (1999).
E.M.A. Martini and I.L. Muller, J. Braz. Chem. Soc. 10, 505 (1999).
M. Pourbaix, Atlas of Electrochemical Equilibria in Aqueous Solutions (Pergamon Press, Oxford, London, U.K. 1966), p. 396.
S.W. Gaarenstroom, J. Vac. Sci. Technol. 16, 600 (1979).
S. Hofmann and J. Steffen, Surf. Interface Anal. 14, 59 (1989).
J.S. Solomon, Thin Solid Films 154, 11 (1987).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rao, R.V.S., Wolff, U., Baunack, S. et al. Stability of the Mg65Y10Cu15Ag10 metallic glass in neutral and weakly acidic media. Journal of Materials Research 18, 97–105 (2003). https://doi.org/10.1557/JMR.2003.0014
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/JMR.2003.0014