Abstract
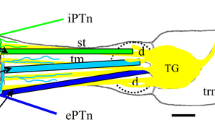
Bending, twitching and quivering are different types of tentacle movements observed during olfactory orientation of the snail. Three recently discovered special muscles, spanning along the length of superior tentacles from the tip to the base, seem to be responsible for the execution of these movements. In this study we have investigated the ultrastructure, contractile properties and protein composition of these muscles. Our ultrastructural studies show that smooth muscle fibers are loosely embedded in a collagen matrix and they are coupled with long sarcolemma protrusions. The muscle fibers apparently lack organized SR and transverse tubular system. Instead subsarcolemmal vesicles and mitochondria have been shown to be possible Ca2+ pools for contraction. It was shown that external Ca2+ is required for contraction elicited by high (40 mM) K+ or 10−4 M ACh. Caffeine (5 mM) induced contraction in Ca2+-free solution suggesting the presence of a substantial intracellular Ca2+ pool. High-resolution electrophoretic analysis of columellar and tentacular muscles did not reveal differences in major contractile proteins, such as actin, myosin and paramyosin. Differences were observed however in several bands representing presumably regulatory enzymes. It is concluded that, the ultrastructural, biochemical and contractile properties of the string muscles support their special physiological function.
Article PDF
Similar content being viewed by others
References
Bone, Q., Inoue, I., Tsutsui, I. (1997) Contraction and relaxation in the absence of a sarcoplasmic reticulum: muscle fibres in the small pelagic tunicate Doliolum. J. Muscle Res. Cell. Motil. 18, 375–380.
Brooks, D. D., Huddart, H., Lennard, R., Hill, R. B. (1990) Calcium utilization in contractures induced by acetylcholine or high-potassium saline in molluscan proboscis muscles. J. Exp. Biol. 149, 379–394.
Chase, R., Hall, B. (1996) Nociceptive inputs to C3, a motoneuron of the tentacle withdrawal reflex in Helix aspersa. J. Comp. Physiol. A 179, 809–818.
Chase, R., Pryer, K., Baker, R., Medison, D. (1978) Responses to conspecific chemical stimuli in the terrestrial snail Achatina fulica. Behav. Biol. 22, 302–315.
Chen, C.-J. (1983) A study of the longitudional body wall muscle of the sea cucumber Sclerodactyla briareus. PhD thesis, University of Rhode Island, USA.
Cianchetti, M., Arienti, A., Follador, M., Mazzolai, B., Dario, P., Laschi, C. (2011) Design concept and validation of a robotic arm inspired by the octopus. Mat. Sci. Eng. 31, 1230–1239.
Dorsett, D. A., Roberts, J. B. (1980) A transverse tubular system and neuromuscular junctions in a molluscan unstriated muscle. Cell Tissue Res. 206, 251–260.
Emery, D. G. (1992) Fine structure of olfactory epithelia of gastropod molluscs. Microsc. Res. Tech. 22, 307–324.
Frescura, M., Hodgson, A. N. (1989) On collagen and its potential role in the columellar muscle of some gastropod molluscs. Suid-Afrikaanse Tydskrif Vir Wetenskap, 85, 613–614.
Gelperin, A. (1974) Olfactory basis of homing behavior in the giant garden slug, Limax maximus. Proc. Nat. Acad. Sci. USA 71, 966–970.
Hernádi, L., Teyke, T. (2012) Novel triplet of flexor muscles in the posterior tentacles of the snail, Helix pomatia. Acta Biol. Hung. 63 (Suppl. 2) 123–128.
Hill, R. B. (2001) Role of Ca2+ in excitation-contraction coupling in echinoderm muscle: comparison with role in other tissues. J. Exp. Biol. 204, 897–908.
Huddart, H., Hunt, S., Oates, K. (1977) Calcium movements during contraction in molluscan smooth muscle, and the loci of calcium binding and release. J. Exp. Biol. 68, 45–56.
Kendrick-Jones, J., Lehman, W., Szent-Györgyi, A. G. (1970) Regulation in molluscan muscles. J. Mol. Biol. 54, 313.
Kier, W. M. (1992) Hydrostatic skeletons and muscular hydrostats. In: Biewener, A. A. (ed.) Biomechanics (Structure and Systems): A Practical Approach. Oxford Univ. Press, New York, pp. 205–231.
Kier, W. M., Schachat, F. H. (1992) Biochemical comparison of fast- and slow-contracting squid muscle. J. Exp. Biol. 168, 41–56.
Kier, W. M., Stella, M. P. (2007) The arrangement and function of Octopus arm musculature and connective tissue. J. Morphol. 268, 831–843.
Laemmili, U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 277, 680–685.
Lemaire, M., Chase, R. (1998) Twitching and quivering of the tentacles during snail olfactory orientation. J. Comp. Physiol. A 182, 81–87.
Lüllmann, H., Sunano, S. (1973) Acetylcholine contracture and excitation-contraction coupling in denervated rat diaphragm muscle. Pflügers Arch. 342, 271–282.
Margulis, B. A., Galaktionov, K. I., Podgornaya, O. I., Pinaev, G. P. (1982) Major contractile proteins of mollusc: tissue polymorphism of actin, tropomyosin and myosin light chains is absent. Comp. Biochem. Physiol. B 72, 473–476.
Moore, P. A., Atema, J., Gerhard, G. A. (1991) Fluid dynamics and microscale chemical movement in the chemosensory appendages of the lobster, Homarus americanus. Chem. Senses 16, 663–674.
Motokawa, T. (1982) Factors regulating the mechanical properties of holothurian dermis. J. Exp. Biol. 99, 29–41.
Peschel, M., Straub, V., Teyke, T. (1996) Consequences of food-attraction conditioning in Helix: a behavioral and electrophysiological study. J. Comp. Physiol. A 178, 317–327.
Prescott, S. A., Gill, N., Chase, R. (1997) Neural circuit mediating tentacle withdrawal in Helix aspersa, with specific reference to the competence of the motor neuron C3. J. Neurophysiol. 78, 2951–2965.
Rigon, F., Manica, G., Guma, F., Achaval, M., Faccioni-Heuser, M. C. (2010) Ultrastructural features of the columellar muscle and contractile protein analyses in different muscle groups of Megalobulimus abbreviatus. Tissue Cell. 42, 53–60.
Somlyo, A. P. (1972) Excitation-contraction coupling in vertebrate smooth muscle: Correlation of ultrastructure with function. Physiologist 15, 338–348.
Treiman, M., Caspersen, C., Christensen, S. B. (1998) A tool coming of age: Thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca2+-ATPases. TIPS 19, 131–135.
Wilkie, I. C. (1996) Mutable collagenous structure or not? A comment on the re-interpretation by del Castillo et al. of the catch mechanism in the sea urchin spine ligament. Biol. Bull. 190, 237–242.
Zakharov, I. S. (1992) Avoidance behavior of the snail. J. Higher Nerv. Act. 42, 1156–1169. (in Russian)
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented during the 12th ISIN Symposium on Invertebrate Neurobiology, August 31–September 4, 2011, Tihany, Hungary.
Rights and permissions
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Krajcs, N., Márk, L., Elekes, K. et al. Morphology, Ultrastructure and Contractile Properties of Muscles Responsible for Superior Tentacle Movements of the Snail. BIOLOGIA FUTURA 63 (Suppl 2), 129–140 (2012). https://doi.org/10.1556/ABiol.63.2012.Suppl.2.17
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1556/ABiol.63.2012.Suppl.2.17