Abstract
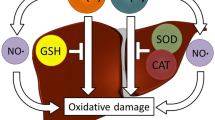
The in vitro effects of alloxan and the product of its reduction dialuric acid (alone or in combination with copper ions) on lipid peroxidation, carbonyl content, GSH level and antioxidant enzyme activities in rat liver and kidney have been studied. The effects of Cu2+/alloxan and Cu2+/dialuric acid were compared with those of Fe3+/alloxan and Fe3+/dialuric acid. Unlike alloxan, dialuric acid increased liver and kidney lipid peroxidation; similar effects were registered in the presence of Fe3+. In the presence of Cu2+/dialuric acid, the lipid peroxidation was strongly inhibited and vice versa - the liver protein oxidation was increased. Alloxan and dialuric acid, as well as their combinations with Fe3+ had no effect on the total GSH level. Both substances did not affect the Cu2+-induced changes in GSH level, glucose-6-phosphate dehydrogenase and gluthatione reductase activities. In contrast, Cu2+ had no effect on dialuric-acid induced changes in gluthatione peroxidase and superoxide dismutase activities. The present in vitro results, concerning the metal dependence of the effects of alloxan and dialuric acid, are a premise for in vivo study of alloxan effects in metal-loaded animals.
Article PDF
Similar content being viewed by others
References
Alexandrova, A., Georgieva, A., Kirkova, M. (2006) Alloxan and dialuric acid. Effects on.OH-provoked degradation of deoxyribose in the presence of different metal ions. C.R. Acad. Bulg. Sci. 59, 305–312.
Alexandrova, A., Kessiova, M., Tsvetanova, E., Kirkova, M. (2006) Alloxan. Effects on O2--provoked inhibition of nitro-blue tetrazolium reduction in the presence of different metal ions. C.R. Acad. Bulg. Sci. 59, 201–206.
Alexandrova, A., Kirkova, M., Russanov, E. (1998) In vitro effects of alloxan-vanadium combination on lipid peroxidation and on antioxidant enzyme activity. Gen. Pharmac. 31, 489–493.
Beauchamp, C., Fridovich, I. (1971) Superoxide dismutase: Improved assays and assay applicable to acrylamide gels. Anal. Biochem. 44, 276–287.
Becker, D. J., Reul, B., Ozcelikay, A. T., Buchet, J. P., Henquin, J. C., Brichard, S. M. (1996) Oral selenates improves glucose homeostasis and partly reverses abnormal expression of liver glycolytic and gluconeogenesis enzymes in diabetic rats. Diabetologia 39, 3–11.
Cartier, P., Leroux, J. P., Marchand, J. Cl. (1967) Techniques de dosage des enzymes glycocytiques tissulaires. Ann. Biol. Clin. 25, 109–136.
Fischer, L. J., Harman, A. W. (1982) Oxygen free radicals and diabetogenic action of alloxan. In: Autor, A. P. (ed.) Pathology of Oxygen. Academic Press, New York. p. 261.
Grankvist, K., Marklund, S. L., Schlin, J., Taljedal, I. B. (1979) Superoxide dismutase, catalase and scavengers of hydroxyl radical protect against the toxic action of alloxan on pancreatic islet cells in vitro. Biochem. J. 182, 17–25.
Gunzler, W. A., Vergin, H., Muller, I., Flohe, L. (1972) Glutathion peroxidase. VI. Die reaction der glutathion peroxidase mit Verschieden hydroperoxiden. Hoppe-Seyler’s Z. Physiol. Chem. 353, 1001–1004.
Halliwell, B., Gutteridge, J. M. C. (1985) Free Radicals in Biology and Medicine. Calderon Press, Oxford.
Halliwell, B., Gutteridge, J. M. C. (1984) Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 219, 1–14.
Halliwell, B., Gutteridge, J. M. C., Aruoma, O. I. (1987) The deoxyribose method: a simple “test-tube” assay for determination of free constants for reactions of hydroxyl radicals. Anal. Biochem. 165, 215–219.
Heikkila, R. E., Winston, B., Cohen, G., Barden, H. (1976) Alloxan-induced diabetes: evidence for hydrohyl radical as a cytotoxic intermediate. Biochem. Pharmac. 25, 1085–1092.
Houee-Levin, C., Gardes-Albert, M., Ferradini, C., Pucheault, J. (1981) Radiolysis study of the allox-an-dialuric acid couple II: the autooxidation of dialuric acid. Radiat. Res. 88, 20–28.
Hunter, F., Gebinski, J., Hoffstein, P., Weinstein, J., Scott, A. (1963) Swelling and lysis of rat liver mitochondria by ferrous ions. J. Biol. Chem. 238, 828–835.
Ishibashi, F., Howard, B. V. (1981) Alloxan and H2O2 action on glucose metabolisis in cultured fibroblasts. Generation of oxygen-containing free radicals as a mechanism of alloxan action. J. Biol. Chem. 256, 12134–12139.
Kirkova, M., Karakashev, P., Russanov, E. (1998) Hydroxyl radicals production in the vanadium ions/dialuric acid systems. Gen. Pharmac. 31, 247–251.
Letelier, M. E., Lepe, A. M., Faundez, M., Salazar, J., Marin, R., Aracena, P., Speiski, H. (2005) Possible mechanisms underlying copper-induced damage in biological membranes leading to cellular toxicity. Chem. Biol. Interact. 151, 71–82.
Lowry, O. H., Rosenbrough, N. J., Farr, A. L., Randal, R. J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–278.
Malaisse, W. J. (1982) Alloxan toxicity to the pancreatic β-cell. A new hypothesis. Biochem. Pharmac. 31, 3527–3534.
McNeill, J. H., Delgatty, H. L. M., Battell, M. L. (1991) Insulin-like effects of sodium selenate in streptozotocin induced diabetic rats. Diabetes 40, 1675–1678.
Meyerovitch, J., Farfel, Z., Sack, J., Schechter, Y. (1987) Oral administration of vanadate normalizes blood glucose levels in streptozotocin-treated rats. J. Biol. Chem. 262, 6658–6662.
Miller, D. M., Grover, A., Nayini, N., Aust, S. D. (1993) Xanthine oxidase- and iron-dependent lipid peroxidation. Arch. Biochem. Biophys. 301, 1–7.
Munday, R. (1988) Effects of transition metals on the reaction rate and on the generation of “active oxygen” species. Biochem. Pharmac. 37, 409–413.
Ozcelikay, A. T., Becker, D. J., Ongemba, L. N., Pottier, A. M., Henquin, J. L., Buchard, S. M. (1996) Improvement of glucose and lipid metabolism in diabetic rats treated with molybdate. Am. J. Physiol. 270, E344-E352.
Pinto, R. E., Bartley, W. (1969) The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic glutathione oxidation in rat liver homogenates. Biochem. J. 112, 109–115.
Reznick, A. Z., Parker, L. (1994) Oxidative damage to ptoteins: Spectrophotometric method for carbonyl assay. Meth. Enzymol. 233, 357–363.
Rodriguez-Gil, J. E., Fernandez-Novell, J. M., Barbera, A., Guinovart, J. J. (2000) Lithium effects on rat glucose metabolism in vivo. Arch. Biochem. Biophys. 375, 377–384.
Schechter, Y. (1990) Insulin-mimetic effects of vanadate. Possible implications for future treatment of diabetes. Diabetes 39, 1–5.
Stadtman, E. R., Oliver, C. N. (1991) Metal-catalyzed oxidation of proteins. J. Biol. Chem. 266, 2005–2008.
Tamura, S., Brown, T. A., Whipple, J. H., Fujita-Yamaguchi, Y., Dubler, R. E., Cheng, K., Larner, J. W. (1984) A novel mechanism for the insulin-like effects of vanadate on glycogen synthase rat adipocytes. J. Biol. Chem. 259, 6650–6658.
Tibaldi, J., Benjamin, J., Cabbat, F. S., Heikkila, R. E. (1979) Protection against alloxan-induced diabetes by various urea derivatives: relationship between protective effects and reactivity with the hydroxyl radical. J. Pharmac. Exp. Ther. 211, 415–418.
Tietze, F. (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 27, 502–522.
Acknowledgement
This study was supported by Grant MU-B-1001 from the National Research Fund, Bulgaria.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Alexandrova, A., Petrov, L., Kessiova, M. et al. In vitro Effects of Alloxan/Copper Combinations on Lipid Peroxidation, Protein Oxidation and Antioxidant Enzymes. BIOLOGIA FUTURA 58, 359–367 (2007). https://doi.org/10.1556/ABiol.58.2007.4.3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1556/ABiol.58.2007.4.3