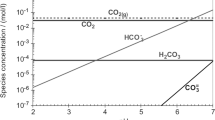
Abstract
In recent years, plant extracts have become the focus of corrosion inhibitor research due to their low toxicity, easy availability and economical preparation. This review presents most of the recent contributions made to the application of plant extracts as corrosion inhibitors for steel in sulphuric acid, as well as in both hydrochloric acid and sulphuric acid. The constituents, properties, adsorption modes and inhibition mechanisms of these natural products are discussed. Evaluation methods and the factors that influence the corrosion-inhibition efficiency of plant extracts are also summarised.
Similar content being viewed by others
References
Abdel-Gaber, A. M., Abd-El-Nabey, B. A., Sidahmed, I. M., El-Zayady, A. M., & Saadawy, M. (2006). Inhibitive action of some plant extracts on the corrosion of steel in acidic media. Corrosion Science, 48, 2765–2779. DOI: 10.1016/j.corsci.2005.09.017.
Ansari, K. R., Quraishi, M. A., & Singh, A. (2015). Isatin derivatives as a non-toxic corrosion inhibitor for mild steel in 20% H2SO4. Corrosion Science, 95, 62–70. DOI: 10.1016/j.corsci.2015.02.010.
Avci, G., & Keleş, Y. (2011). Aqueous extract of Acacia cyanophylla leaves as environmentally friendly inhibitor for mild steel corrosion in 1 M H2SO4 solution. Surface and Interface Analysis, 43, 1311–1317. DOI: 10.1002/sia.3714.
Behpour, M., Ghoreishi, S. M., Khayatkashani, M., & Soltani, N. (2012). Green approach to corrosion inhibition of mild steel in two acidic solutions by the extract of Punica granatum peel and main constituents. Materials Chemistry and Physics, 131, 621–633. DOI: 10.1016/j.matchemphys.2011.10.027.
Benali, O., Selles, C., & Salghi, R. (2014). Inhibition of acid corrosion of mild steel by Anacyclus pyrethrum L. extracts. Research on Chemical Intermediates, 40, 259–268. DOI: 10.1007/s11164–012–0960–8.
Benmenine, A. E. K., Ouahrani, M. R., Gherraf, N., Sekirifa, M. L., & Baameur, L. (2011). Potentiodynamic investigation of the anticorrosive action of Cotula cinerae extracts on mild steel X52 in 20% H2SO4 solution. Journal of Chemical and Pharmaceutical Research, 3(3), 666–669.
Benmenine, A. E. K., Ouahrani, M. R., Gherraf, N., Sekirifa, M. L., & Baameur, L. (2014). Anticorrosive action study of Retama retam extracts on mild steel X52 in 20% H2SO4 solution. Energy Procedia, 50, 401–405. DOI: 10.1016/j.egypro.2014.06.048.
Cang, H., Fei, Z. H., Xiao, H. R., Huang, J. L., & Xu, Q. (2012). Inhibition effect of reed leaves extract on steel in hydrochloric acid and sulphuric acid solutions. International Journal of Electrochemical Science, 7, 8869–8882.
Cardozo da Rocha, J., da Cunha Ponciano Gomes, J. A., & D’Elia, E. (2010). Corrosion inhibition of carbon steel in hydrochloric acid solution by fruit peel aqueous extracts. Corrosion Science, 52, 2341–2348. DOI: 10.1016/j.corsci.2010.03.033.
Chidiebere, M. A., Ogukwe, C. E., Oguzie, K. L., Eneh, C. N., & Oguzie, E. E. (2012). Corrosion inhibition and adsorption behavior of Punica granatum extract on mild steel in acidic environments: Experimental and theoretical studies. Industrial & Engineering Chemistry Research, 51, 668–677. DOI: 10.1021/ie201941f.
Cristofari, G., Znini, M., Majidi, L., Bouyanzer, A., Al-Deyab, S. S., Paolini, J., Hammouti, B., & Costa, J. (2011). Chemical composition and anti-corrosive activity of Pulicaria mauritanica essential oil against the corrosion of mild steel in 0.5 M H2SO4. International Journal of Electrochemical Science, 6, 6699–6717.
Dar, M. A. (2011). A review: plant extracts and oils as corrosion inhibitors in aggressive media. Industrial Lubrication and Tribology, 63, 227–233. DOI: 10.1108/00368791111140431.
Deng, S. D., & Li, X. H. (2012). Inhibition by Ginkgo leaves extract of the corrosion of steel in HCl and H2SO4 solutions. Corrosion Science, 55, 407–415. DOI: 10.1016/j.corsci.2011.11.005.
Eddy, N. O., Odoemelam, S. A., & Odiongenyi, A. O. (2009). Joint effect of halides and ethanol extract of Lasianthera africana on inhibition of corrosion of mild steel in H2SO4. Journal of Applied Electrochemistry, 39, 849–857. DOI: 10.1007/s10800–008–9731-z.
Eddy, N. O., & Ebenso, E. E. (2010). Corrosion inhibition and adsorption properties of ethanol extract of Gongronema latifolium on mild steel in H2SO4. Pigment & Resin Technology, 39, 77–83. DOI: 10.1108/03699421011028653.
Eddy, N. O., & Odiongenyi, A. O. (2010). Corrosion inhibition and adsorption properties of ethanol extract of Heinsia crinata on mild steel in H2SO4. Pigment & Resin Technology, 39, 288–295. DOI: 10.1108/03699421011076407.
Eduok, U. M., Umoren, S. A., & Udoh, A. P. (2012). Synergistic inhibition effects between leaves and stem extracts of Sida acuta and iodide ion for mild steel corrosion in 1 M H2SO4 solutions. Arabian Journal of Chemistry, 5, 325–337. DOI: 10.1016/j.arabjc.2010.09.006.
Elhosary, A. A., Saleh, R. M., & Shams El Din, A. M. (1972). Corrosion inhibition by naturally occurring substance—I. The effect of Hibiscus subdariffa (karkade) extract on the dissolution of Al and Zn. Corrosion Science, 12, 897–904. DOI: 10.1016/s0010–938x(72)80098–2.
Ezeoke, A. U., Obi-Egbedi, N. O., Adeosun, C. B., & Adeyemi, O. G. (2012). Synergistic effect of leaf extracts of Cordia sebestena L. and iodide ions on the corrosion inhibition of mild steel in sulphuric acid. International Journal of Electrochemical Science, 7, 5339–5355.
Faustin, M., Maciuk, A., Salvin, P., Roos, C., & Lebrini, M. (2015). Corrosion inhibition of C38 steel by alkaloids extract of Geissospermum laeve in 1 M hydrochloric acid: Electrochemical and phytochemical studies. Corrosion Science, 92, 287–300. DOI: 10.1016/j.corsci.2014.12.005.
Gaur, B., Singh, T. B., & Singh, D. D. N. (1996). Beneficial role of chloride ions during pickling of steel in sulfuric acid. Corrosion, 52, 154–159. DOI: 10.5006/1.3292106.
Gece, G. (2008). The use of quantum chemical methods in corrosion inhibitor studies. Corrosion Science, 50, 2981–2992. DOI: 10.1016/j.corsci.2008.08.043.
Hooshmand Zaferani, S., Sharifi, M., Zaarei, D., & Shishesaz, M. R. (2013). Application of eco-friendly products as corrosion inhibitors for metals in acid pickling processes — A review. Journal of Environmental Chemical Engineering, 1, 652–657. DOI: 10.1016/j.jece.2013.09.019.
Ikeuba, A. I., Okafor, P. C., Ekpe, U. J., & Ebenso, E. E. (2013). Alkaloid and non-alkaloid ethanolic extracts from seeds of Garcinia kola as green corrosion inhibitors of mild steel in H2SO4 solution. International Journal of Electrochemical Science, 8, 7455–7467.
Ikpi, M. E., Udoh, I. I., Okafor, P. C., Ekpe, U. J., & Ebenso, E. E. (2012). Corrosion inhibition and adsorption behaviour of extracts from Piper guineensis on mild steel corrosion in acid media. International Journal of Electrochemical Science, 7, 12193–12206.
Ji, G., Dwivedi, P., Sundaram, S., & Prakash, R. (2013). Inhibitive effect of Chlorophytum borivilianum root extract on mild steel corrosion in HCl and H2SO4 solutions. Industrial & Engineering Chemistry Research, 52, 10673–10681. DOI: 10.1021/ie4008387.
Kamal, C., & Sethuraman, M. G. (2013). Hydroclathrus clathratus marine alga as a green inhibitor of acid corrosion of mild steel. Research on Chemical Intermediates, 39, 3813–3828. DOI: 10.1007/s11164–012–0883–4.
Krishnaveni, K., Ravichandran, J., & Selvaraj, A. (2014). Inhibition of mild steel corrosion by Morinda tinctoria leaves extract in sulphuric acid medium. Ionics, 20, 115–126. DOI: 10.1007/s11581-013-0954-6.
Krishnegowda, P. M., Venkatesha, V. T., Krishnegowda, P. K. M., & Shivayogiraju, S. B. (2013). Acalypha torta leaf extract as green corrosion inhibitor for mild steel in hydrochloric acid solution. Industrial & Engineering Chemistry Research, 52, 722–728. DOI: 10.1021/ie3018862.
Li, X. H., Deng, S. D., & Fu, H. (2012). Inhibition of the corrosion of steel in HCl, H2SO4 solutions by bamboo leaf extract. Corrosion Science, 62, 163–175. DOI: 10.1016/j.corsci.2012.05.008.
Loganayagi, C., Kamal, C., & Sethuraman, M. G. (2014). Opuntiol: An active principle of Opuntia elatior as an ecofriendly inhibitor of corrosion of mild steel in acid medium. ACS Sustainable Chemistry & Engineering, 2, 606–613. DOI: 10.1021/sc4003642.
Loto, C. A. (2005). Inhibition of cashew juice on the corrosion of mild steel in sulphuric acid. Corrosion Prevention & Control, 52(1), 13–21.
Martinez, S., & Stern, I. (2001). Inhibitory mechanism of low-carbon steel corrosion by mimosa tannin in sulphuric acid solutions. Journal of Applied Electrochemistry, 31, 973–978. DOI: 10.1023/a: 1017989510605.
Martinez, S., & Stern, I. (2002). Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in the low carbon steel/mimosa tannin/sulphuric acid system. Applied Surface Science, 199, 83–89. DOI: 10.1016/s0169-4332(02)00546–9.
Martinez, S., & Stagljar, I. (2003). Correlation between the molecular structure and the corrosion inhibition efficiency of chestnut tannin in acidic solutions. Journal of Molecular Structure: THEOCHEM, 640, 167–174. DOI: 10.1016/j.theochem.2003.08.126.
Mourya, P., Banerjee, S., & Singh, M. M. (2014). Corrosion inhibition of mild steel in acidic solution by Tagetes erecta (Marigold flower) extract as a green inhibitor. Corrosion Science, 85, 352–363. DOI: 10.1016/j.corsci.2014.04.036.
Muthukrishnan, P., Jeyaprabha, B., & Prakash, P. (2013a). Corrosion inhibition and adsorption behavior of Setaria verticillata leaf extract in 1M sulphuric acid. Journal of Materials Engineering and Performance, 22, 3792–3800. DOI: 10.1007/s11665-013-0700-2.
Muthukrishnan, P., Jeyaprabha, B., & Prakash, P. (2013b). Adsorption and corrosion inhibiting behavior of Lannea coro-mandelica leaf extract on mild steel corrosion. Arabian Journal of Chemistry. DOI: 10.1016/j.arabjc.2013.08.011.
Muthukrishnan, P., Jeyaprabha, B., & Prakash, P. (2013c). Corrosion inhibition of Leucaena leucocephala pod on mild steel in sulphuric acid solution. Acta Metallurgica Sinica, 26, 416–424. DOI: 10.1007/s40195-013-0082-3.
Muthukrishnan, P., Kumar, K. S., Jeyaprabha, B., & Prakash, P. (2014). Anticorrosive activity of Kigelia pinnata leaves extract on mild steel in acidic media. Metallurgical and Materials Transactions A, 45, 4510–4524. DOI: 10.1007/s11661-014–2366–2.
Noor, E. A. (2011). The impact of some factors on the inhibitory action of Radish seeds aqueous extract for mild steel corrosion in 1 M H2SO4 solution. Materials Chemistry and Physics, 131, 160–169. DOI: 10.1016/j.matchemphys.2011.08.001.
Obot, I. B., Ebenso, E. E., & Gasem, Z. M. (2012). Eco-friendly corrosion inhibitors: Adsorption and inhibitive action of ethanol extracts of Chlomolaena odorata L. for the corrosion of mild steel in H2SO4 solutions. International Journal of Electrochemical Science, 7, 1997–2008.
Odewunmi, N. A., Umoren, S. A., & Gasem, Z. M. (2015). Utilization of watermelon rind extract as a green corrosion inhibitor for mild steel in acidic media. Journal of Industrial and Engineering Chemistry, 21, 239–247. DOI: 10.1016/j.jiec.2014.02.030.
Oguzie, E. E. (2006a). Adsorption and corrosion inhibitive properties of Azadirachta indica in acid solutions. Pigment & Resin Technology, 35, 334–340. DOI: 10.1108/036994206107 11335.
Oguzie, E. E. (2006b). Studies on the inhibitive effect of Occimum viridis extract on the acid corrosion of mild steel. Materials Chemistry and Physics, 99, 441–446. DOI: 10.1016/j.matchemphys.2005.11.018.
Oguzie, E. E. (2008). Evaluation of the inhibitive effect of some plant extracts on the acid corrosion of mild steel. Corrosion Science, 50, 2993–2998. DOI: 10.1016/j.corsci.2008.08.004.
Oguzie, E. E., Enenebeaku, C. K., Akalezi, C. O., Okoro, S. C., Ayuk, A. A., & Ejike, E. N. (2010). Adsorption and corrosion-inhibiting effect of Dacryodis edulis extract on low-carbon-steel corrosion in acidic media. Journal of Colloid and Interface Science, 349, 283–292. DOI: 10.1016/j.jcis.2010.05.027.
Oguzie, E. E., Adindu, C. B., Enenebeaku, C. K., Ogukwe, C. E., Chidiebere, M. A., & Oguzie, K. L. (2012a). Natural products for materials protection: Mechanism of corrosion inhibition of mild steel by acid extracts of Piper guineense. The Journal of Physical Chemistry C, 116, 13603–13615. DOI: 10.1021/jp300791s.
Oguzie, E. E., Ogukwe, C. E., Ogbulie, J. N., Nwanebu, F. C., Adindu, C. B., Udeze, I. O., Oguzie, K. L., & Eze, F. C. (2012b). Broad spectrum corrosion inhibition: corrosion and microbial (SRB) growth inhibiting effects of Piper guineense extract. Journal of Materials Science, 47, 3592–3601. DOI: 10.1007/s10853-011-6205-1.
Oguzie, E. E., Iheabunike, Z. O., Oguzie, K. L., Ogukwe, C. E., Chidiebere, M. A., Enenebeaku, C. K., & Akalezi, C. O. (2013). Corrosion inhibiting effect of Aframomum melegueta extracts and adsorption characteristics of the active constituents on mild steel in acidic media. Journal of Dispersion Science and Technology, 34, 516–527. DOI: 10.1080/01932691.2012.682008.
Okafor, P. C., & Ebenso, E. E. (2007). Inhibitive action of Carica papaya extracts on the corrosion of mild steel in acidic media and their adsorption characteristics. Pigment & Resin Technology, 36, 134–140. DOI: 10.1108/03699420710748992.
Okafor, P. C., Osabor, V. I., & Ebenso, E. E. (2007). Eco-friendly corrosion inhibitors: inhibitive action of ethanol extracts of Garcinia kola for the corrosion of mild steel in H2SO4 solutions. Pigment & Resin Technology, 36, 299–305. DOI: 10.1108/03699420710820414.
Okafor, P. C., Ikpi, M. E., Uwah, I. E., Ebenso, E. E., Ekpe, U. J., & Umoren, S. A. (2008). Inhibitory action of Phyllanthus amarus extracts on the corrosion of mild steel in acidic media. Corrosion Science, 50, 2310–2317. DOI: 10.1016/j.corsci.2008.05.009.
Okafor, P. C., Uwah, I. E., Ekerenam, O. O., & Ekpe, U. J. (2009). Combretum bracteosum extracts as eco-friendly corrosion inhibitor for mild steel in acidic medium. Pigment & Resin Technology, 38, 236–241. DOI: 10.1108/036994209109 73323.
Okafor, P. C., Ebenso, E. E., & Ekpe, U. J. (2010). Azadirachta indica extracts as corrosion inhibitor for mild steel in acid medium. International Journal of Electrochemical Science, 5, 978–993.
Okafor, P. C., Ikpi, M. E., Ekanem, U. I., & Ebenso, E. E. (2013). Effects of extracts from Nauclea latifolia on the dissolution of carbon steel in H2SO4 solutions. International Journal of Electrochemical Science, 8, 12278–12286.
Okeniyi, J. O., Loto, C. A., & Popoola, A. P. I. (2014). Rhizophora mangle L. effects on steel-reinforced concrete in 0.5 M H2SO4: Implications for corrosion-degradation of wind-energy structures in industrial environments. Energy Procedia, 50, 429–436. DOI: 10.1016/j.egypro.2014.06.052.
Patel, N. S., Jauhariand, S., Mehta, G. N., Al-Deyab, S. S., Warad, I., & Hammouti, B. (2013). Mild steel corrosion inhibition by various plant extracts in 0.5 M sulphuric acid. International Journal of Electrochemical Science, 8(2), 2635–2655.
Patel, N. S., & Snita, D. (2014). Ethanol extracts of Hemidesmus indicus leaves as eco-friendly inhibitor of mild steel corrosion in H2SO4 medium. Chemical Papers, 68, 1747–1754. DOI: 10.2478/s11696–014–0625–8.
Popoola, A. P. I., Abdulwahab, M., & Fayomi, O. S. I. (2012). Corrosion inhibition of mild steel in Sesamum indicum-2M HCl/H2SO4 interface. International Journal of Electrochemical Science, 7, 5805–5816.
Popova, A., Sokolova, E., Raicheva, S., & Christov, M. (2003). AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives. Corrosion Science, 45, 33–58. DOI: 10.1016/s0010-938x(02)00072-0.
Quraishi, M. A., Singh, A., Singh, V. K., Yadav, D. K., & Singh, A. K. (2010). Green approach to corrosion inhibition of mild steel in hydrochloric acid and sulphuric acid solutions by the extract of Murraya koenigii leaves. Materials Chemistry and Physics, 122, 114–122. DOI: 10.1016/j.matchemphys.2010.02.066.
Raja, P. B., & Sethuraman, M. G. (2008a). Natural products as corrosion inhibitor for metals in corrosive media—A review. Materials Letters, 62, 113–116. DOI: 10.1016/j.matlet.2007.04.079.
Raja, P. B., & Sethuraman, M. G. (2008b). Inhibitive effect of black pepper extract on the sulphuric acid corrosion of mild steel. Materials Letters, 62, 2977–2979. DOI: 10.1016/j.matlet.2008.01.087.
Raja, P. B., & Sethuraman, M. G. (2010). Studies on the inhibition of mild steel corrosion by Rauvolfia serpentina in acid media. Journal of Materials Engineering and Performance, 19, 761–766. DOI: 10.1007/s11665-009-9541-4.
Raja, P. B., Rahim, A. A., Osman, H., & Awang, K. (2010). Inhibitory effect of Kopsia singapurensis extract on the corrosion behavior of mild steel in acid media. Acta Physico-Chimica Sinica, 26, 2171–2176.
de Oliveira Ramos, R., Battistin, A., & Simoes Goncalves, R. (2012). Alcoholic Mentha extracts as inhibitors of low-carbon steel corrosion in aqueous medium. Journal of Solid State Electrochemistry, 16, 747–752. DOI: 10.1007/s10008-011–1422–8.
Saleh, R. M., Ismail, A. A., & El Hosary, A. A. (1982). Corrosion inhibition by naturally occurring substances: VII. The effect of aqueous extracts of some leaves and fruit-peels on the corrosion of steel, Al, Zn and Cu in acids. British Corrosion Journal, 17, 131–135. DOI: 10.1179/000705982798274345.
Singh, A., & Quraishi, M. A. (2015). The extract of Jamun (Syzygium cumini) seed as green corrosion inhibitor for acid media. Research on Chemical Intermediates, 41, 2901–2914. DOI: 10.1007/s11164-013-1398-3.
Stoyanova, A. E., Sokolova, E. I., & Raicheva, S. N. (1997). The inhibition of mild steel corrosion in 1 M HCl in the presence of linear and cyclic thiocarbamides—Effect of concentration and temperature of the corrosion medium on their protective action. Corrosion Science, 39, 1595–1604. DOI: 10.1016/s0010-938x(97)00063-2.
Suleiman, I. Y., Oloche, O. B., & Yaro, S. A. (2013). The development of a mathematical model for the prediction of corrosion rate behaviour for mild steel in 0.5 M sulphuric acid. ISRN Corrosion, 2013, 710579. DOI: 10.1155/2013/710579.
Subramanian, A. K., Arumugam, S., Mallaiya, K., & Subramaniam, R. (2013). Synergistic effect on corrosion inhibiton efficiency of Ginger affinale extract in controlling corrosion of mild steel in acid medium. Journal of the Korean Chemical Society, 57, 672–676. DOI: 10.5012/jkcs.2013.57.6.672.
Umoren, S. A., Obot, I. B., & Obi-Egbedi, N. O. (2009). Raphia hookeri gum as a potential eco-friendly inhibitor for mild steel in sulfuric acid. Journal of Materials Science, 44, 274–279. DOI: 10.1007/s10853-008-3045-8.
Umoren, S. A., & Ekanem, U. F. (2010). Inhibition of mild steel corrosion in H2SO4 using exudate gum from Pachylobus edulis and synergistic potassium halide additives. Chemical Engineering Communications, 197, 1339–1356. DOI: 10.1080/00986441003626086.
Umoren, S. A., Eduok, U. M., Solomon, M. M., & Udoh, A. P. (2011). Corrosion inhibition by leaves and stem extracts of Sida acuta for mild steel in 1 M H2SO4 solutions investigated by chemical and spectroscopic techniques. Arabian Journal of Chemistry. DOI: 10.1016/j.arabjc.2011.03.008.
Umoren, S. A., Gasem, Z. M., & Obot, I. B. (2013). Natural products for material protection: Inhibition of mild steel corrosion by date palm seed extracts in acidic media. Industrial & Engineering Chemistry Research, 52, 14855–14865. DOI: 10.1021/ie401737u.
Umoren, S. A., Obot, I. B., Israel, A. U., Asuquo, P. O., Solomon, M. M., Eduok, U. M., & Udoh, A. P. (2014a). Inhibition of mild steel corrosion in acidic medium using coconut coir dust extracted from water and methanol as solvents. Journal of Industrial and Engineering Chemistry, 20, 36123622. DOI: 10.1016/j.jiec.2013.12.056.
Umoren, S. A., Solomon, M. M., Eduok, U. M., Obot, I. B., & Israel, A. U. (2014b). Inhibition of mild steel corrosion in H2SO4 solution by coconut coir dust extract obtained from different solvent systems and synergistic effect of iodide ions: Ethanol and acetone extracts. Journal of Environmental Chemical Engineering, 2, 1048–1060. DOI: 10.1016/j.jece.2014.03.024.
Zelinka, S. L., & Stone, D. S. (2011). The effect of tannins and pH on the corrosion of steel in wood extracts. Materials and Corrosion, 62, 739–744. DOI: 10.1002/maco.201005845.
Znini, M., Majidi, L., Laghchimi, A., Paolini, J., Hammouti, B., Costa, J., Bouyanzer, A., & Al-Deyab, S. S. (2011). Chemical composition and anticorrosive activity of Warionia saharea essential oil against the corrosion of mild steel in 0.5 M H2SO4. International Journal of Electrochemical Science, 6, 5940–5595.
Znini, M., Paolini, J., Majidi, L., Desjobert, J. M., Costa, J., Lahhit, N., & Bouyanzer, A. (2012). Evaluation of the inhibitive effect of essential oil of Lavandula multifida L., on the corrosion behavior of C38 steel in 0.5 M H2SO4 medium. Research on Chemical Intermediates, 38, 669–683. DOI: 10.1007/s11164-011-0407-7.
Zucchi, F., Trabanelli, G., & Brunoro, G. (1994). Iron corrosion inhibition in hot 4 M HCl solution by i-cinnamaldehyde and its structure-related compounds. Corrosion Science, 36, 1683–1690. DOI: 10.1016/0010-938x(94)90123-6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mo, S., Luo, HQ. & Li, NB. Plant extracts as “green” corrosion inhibitors for steel in sulphuric acid. Chem. Pap. 70, 1131–1143 (2016). https://doi.org/10.1515/chempap-2016-0055
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/chempap-2016-0055