Abstract
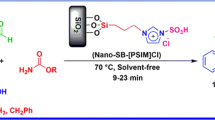
A novel silica-bonded propyl-N-sulfamic acid nanocatalyst (NHSO3H-KIT-5) supported on modified silica mesopore KIT-5 as an organic-inorganic hybrid with high specific surface area was successfully prepared. The 3-aminopropyltriethoxysilane (APTES) on KIT-5 was reacted with chlorosulfonic acid and accurately characterized by the FT-IR, XRD, SEM, EDAXS, and TGA techniques. This heterogeneous and recyclable catalyst catalyzed one pot, multicomponent condensation of benzil, aromatic aldehydes, and ammonium acetate in the presences of 0.05 g of nanocatalyst under solvent-free conditions to afford triaryl-imidazoles in excellent yields. This catalyst showed high catalytic activity under green conditions. This reaction was performed under open air conditions and required no special reaction conditions or chromatographic separation for purification.
Similar content being viewed by others
References
Bader, R. F. W., & Essén, H. (1984). The characterization of atomic interactions. The Journal of Chemical Physics, 80, 1943–1960. DOI: 10.1063/1.446956.
Bader, R. F. W. (1990). Atoms in molecules: A quantum theory. Oxford, UK: Oxford University Press.
Bader, R. F. W. (2000). AIM2000 program, ver. 2.0 [computer software]. Hamilton, Canada: McMaster University.
Bakavoli, M., Eshghi, H., Rahimizadeh, M., Housaindokht, M. R., Mohammadi, A., & Monhemi, H. (2015). Deep eutectic solvent for multi-component reactions: a highly efficient and reusable acidic catalyst for synthesis of 2,4,5-triaryl-1Himidazoles. Research on Chemical Intermediates, 41, 34973505. DOI: 10.1007/s11164-013-1467-7.
Bamoharram, F. F., Heravi, M. M., Roshani, M., Jahanagir, M., & Gharib, A. (2007). Effective direct esterification of butanol by eco-friendly Preyssler catalyst, [NaP5W30O110]14−. Journal of Molecular Catalysis A: Chemical, 271, 126–130. DOI: 10.1016/j.molcata.2007.02.034.
Blotny, G. (2006). Recent applications of 2,4,6-trichloro-1,3,5-triazine and its derivatives in organic synthesis. Tetrahedron, 62, 9507–9522. DOI: 10.1016/j.tet.2006.07.039.
Davoodnia, A., Heravi, M. M., Safavi-Rad, Z., & TavakoliHoseini, N. (2010). Green, one-pot, solvent-free synthesis of 1,2,4,5-tetrasubstituted imidazoles using a Brønsted acidic ionic liquid as novel and reusable catalyst. Synthetic Communications, 40, 2588–2597. DOI: 10.1080/00397910903289271.
Fan, X. S., Qu, Y. Y., Wang, Y. Y., Zhang, X. Y., & Wang, J. J. (2010). Ru(III)-catalyzed oxidation of homopropargyl alcohols in ionic liquid: an efficient and green route to 1,2allenic ketones. Tetrahedron Letters, 51, 2123–2126. DOI: 10.1016/j.tetlet.2010.02.053.
Guisnet, M., Barrault, J., Bouchoule, C., Duprez, D., Montassier, C., & Pérot, G. (Eds.) (1988). Heterogeneous catalysis and fine chemicals (Studies in surface science, Vol. 41). Amsterdam, The Netherlands: Elsevier.
Hamon, F., Prié, G., Lecornué, F., & Papot, S. (2009). Cyanuric chloride: an efficient reagent for the Lossen rearrangement. Tetrahedron Letter, 50, 6800–6802. DOI: 10.1016/j.tetlet.2009.09.115.
Hashemi, E., Beheshtiha, Y. S., Ahmadi, S., & Heravi, M. M. (2014). In situ prepared CuI nanoparticles on modified poly(styrene-co-maleic anhydride): an efficient and recyclable catalyst for the azide—alkyne click reaction in water. Transition Metal Chemistry, 39, 593–601. DOI: 10.1007/s11243014-9838-5.
Heravi, M. M., Montazeri, N., Rahmizadeh, M., Bakavoli, M., & Ghassemzadeh, M. (2000). Zeolite-induced heterocyclization: a superior method of synthesis of condensed imidazoles. Journal of Chemical Research, 2000, 584–585. DOI: 10.3184/030823400103166274.
Heravi, M. M., Rajabzadeh, G., Bamoharram, F. F., & Seifi, N. (2006a). An eco-friendly catalytic route for synthesis of 4amino-pyrazolo[3,4-d]pyrimidine derivatives by Keggin heteropolyacids under classical heating and microwave irradiation. Journal of Molecular Catalysis A: Chemical, 256, 238–241. DOI: 10.1016/j.molcata.2006.04.016.
Heravi, M. M., Tajbakhsh, M., Ahmadi, A. N., & Mohajerani, B. (2006b). Zeolites. Efficient and eco-friendly catalysts for the synthesis of benzimidazoles. Monatshefte für Chemie Chemical Monthly, 137, 175–179. DOI: 10.1007/s00706-0050407-7.
Heravi, M. M., Bakhtiari, K., Oskooie, H. A., & Taheri, S. (2007a). Synthesis of 2,4,5-triaryl-imidazoles catalyzed by NiCl2⊙6H2O under heterogeneous system. Journal of Molecular Catalysis A: Chemical, 263, 279–281. DOI: 10.1016/j.molcata.2006.08.070.
Heravi, M. M., Bakhtiari, K., Daroogheha, Z., & Bamoharram, F. F. (2007b). An efficient synthesis of 2,4,6triarylpyridines catalyzed by heteropolyacid under solventfree conditions. Catalysis Communications, 8, 1991–1994. DOI: 10.1016/j.catcom.2007.03.028.
Heravi, M. M., Bakhtiari, K., Zadsirjan, V., Bamoharram, F. F., & Heravi, O. M. (2007c). Aqua mediated synthesis of substituted 2-amino-4H-chromenes catalyzed by green and reusable Preyssler heteropolyacid. Bioorganic & Medicinal Chemistry Letters, 17, 4262–4265. DOI: 10.1016/j.bmcl.2007.05.023.
Heravi, M. M., Derikvand, F., & Bamoharram, F. F. (2007d). Highly efficient, four-component one-pot synthesis of tetrasubstituted imidazoles using Keggin-type heteropolyacids as green and reusable catalysts. Journal of Molecular Catalysis A: Chemical, 263, 112–114. DOI: 10.1016/j.molcata.2006.08.048.
Heravi, M. M., Bakhtiari, K., Fatehi, A., & Bamoharram, F. F. (2008a). A convenient synthesis of bis(indolyl)methanes catalyzed by diphosphooctadecatungstic acid. Catalysis Communications, 9, 289–292. DOI: 10.1016/j.catcom.2007.07.039.
Heravi, M. M., Derikvand, F., & Haghighi, M. (2008b). Highly efficient, four component, one-pot synthesis of tetrasubstituted imidazoles using a catalytic amount of FeCl3⊙6H2O. Monatshefte fur Chemie Chemical Monthly, 139, 31–33. DOI: 10.1007/s00706-007-0736-9.
Heravi, M. M., & Sadjadi, S. (2009). Recent developments in use of heteropolyacids, their salts and polyoxometalates in organic synthesis. Journal of the Iranian Chemical Society, 6, 1–54. DOI: 10.1007/bf03246501.
Heravi, M. M., Sadjadi, S., Sadjadi, S., Oskooie, H. A., & Bamoharram, F. F. (2009a). Rapid and efficient synthesis of 4(3H)-quinazolinones under ultra sonic irradiation using silica-supported Preyssler nano particles. Ultrasonics Sonochemistry, 16, 708–710. DOI: 10.1016/j.ultsonch.2009.02.010.
Heravi, M. M., Sadjadi, S., Sadjadi, S., Oskooie, H. A., & Bamoharram, F. F. (2009b). A convenient synthesis of bis(indolyl)alkanes under ultra sonic irradiation using silicasupported Preyssler nano particles. Ultrasonics Sonochemistry, 16, 718–720. DOI: 10.1016/j.ultsonch.2009.04.004.
Heravi, M. M., Tavakoli-Hoseini, N., & Bamoharram, F. F. (2010). Silica-supported Preyssler nano particles: a green, reusable and highly efficient heterogeneous catalyst for the synthesis of carbamatoalkyl naphthols. Green Chemistry Letters and Reviews, 3, 263–267. DOI: 10.1080/1751825100374 9387.
Heravi, M. M., & Alishiri, T. (2012). Application of nanomaterials in heterocyclic chemistry. Heterocycles, 85, 545–586. DOI: 10.3987/rev-11-725.
Heravi, M. M., Hashemi, E., Beheshtiha, Y. S., Ahmadi, S., & Hosseinnejad, T. (2014). PdCl2 on modified poly(styrene-comaleic anhydride): A highly active and recyclable catalyst for the Suzuki-Miyaura and Sonogashira reactions. Journal of Molecular Catalysis A: Chemical, 394, 74–82. DOI: 10.1016/j.molcata.2014.07.001.
Heravi, M. M., Daraie, M., & Zadsirjan, V. (2015). Current advances in the synthesis and biological potencies of triand tetra-substituted 1H-imidazoles. Molecular Diversity, 19, 577–623. DOI: 10.1007/s11030-015-9590-6.
Karimi, A. R., Alimohammadi, Z., & Amini, M. M. (2010). Wells-Dawson heteropolyacid supported on silica: a highly efficient catalyst for synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles. Molecular Diversity, 14, 635–641. DOI: 10.1007/s11030-009-9197-x.
Khorami, F., & Shaterian, H. R. (2014). Silica-bonded propylpiperazine-N-sulfamic acid as recyclable solid acid catalyst for preparation of 2-amino-3-cyano-4-aryl-5,10-dioxo5,10-dihydro-4H-benzo[g]chromenes and hydroxy-substituted naphthalene-1,4-dione derivatives. Chinese Journal of Catalysis, 35, 242–246. DOI: 10.1016/s1872–2067(12)60761-x.
Khosropour, A. R. (2008). Ultrasound-promoted greener synthesis of 2,4,5-trisubstituted imidazoles catalyzed by Zr(acac)4 under ambient conditions. Ultrasonics Sonochemistry, 15, 659–664. DOI: 10.1016/j.ultsonch.2007.12.005.
Kidwai, M., Mothsra, P., Bansal, V., Somvanshi, R. K., Ethayathulla, A. S., Dey, S., & Singh, T. P. (2007). One-pot synthesis of highly substituted imidazoles using molecular iodine: A versatile catalyst. Journal of Molecular Catalysis A: Chemical, 265, 177–182. DOI: 10.1016/j.molcata.2006.10.009.
Kleitz, F., Liu, D. N., Anilkumar, G. M., Park, I. S., Solovyov, L. A., Shmakov, A. N., & Ryoo, R. (2003). Large cage facecentered-cubic Fm3m mesoporous silica: Synthesis and structure. The Journal of Physical Chemistry B, 107, 14296–14300. DOI: 10.1021/jp036136b.
Lee, C. T., Yang, W. T., & Parr, R. G. (1988). Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Physical Review B, 37, 785–789. DOI: 10.1103/PhysRevB.37.785.
Li, B. Z., Gu, Q., He, Y. H., Zhao, T. Q., Wang, S. J., Kang, J., & Zhang, Y. M. (2012). Facile synthesis of trisubstituted imidazoles from 1,2-di(furan-2-yl)-2-oxoethyl carboxylates and their chemiluminescence. Comptes Rendus Chimie, 15, 784–792. DOI: 10.1016/j.crci.2012.06.005.
Liu, L., Ma, J. J., Ji, L. Y., & Wei, Y. Y. (2008). Molecular sieve promoted copper catalyzed aerobic oxidation of alcohols to corresponding aldehydes or ketones. Journal of Molecular Catalysis A: Chemical, 291, 1–4. DOI: 10.1016/j.molcata.2008.06.001.
Mahdavinia, G. H., Amani, A. M., & Sepehrian, H. (2012). MCM-41-SO3H as a highly efficient sulfonic acid nanoreactor for the rapid and green synthesis of some novel highly substituted imidazoles under solvent-free condition. Chinese Journal of Chemistry, 30, 703–708. DOI: 10.1002/cjoc.201280008.
Mannam, S., & Sekar, G. (2008). CuCl catalyzed oxidation of aldehydes to carboxylic acids with aqueous tert-butyl hydroperoxide under mild conditions. Tetrahedron Letters, 49, 1083–1086. DOI: 10.1016/j.tetlet.2007.11.198.
Martín, S. E., & Garrone, A. (2003). Efficient solvent-free iron(III) catalyzed oxidation of alcohols by hydrogen peroxide. Tetrahedron Letters, 44, 549–552. DOI: 10.1016/s00404039(02)02569–8.
Mirsafaei, R., Heravi, M. M., Ahmadi, S., Moslemin, M. H., & Hosseinnejad, T. (2015). In situ prepared copper nanoparticles on modified KIT-5 as an efficient recyclable catalyst and its applications in click reactions in water. Journal of Molecular Catalysis A: Chemical, 402, 100–108. DOI: 10.1016/j.molcata.2015.03.006.
Moghaddas, M., Davoodnia, A., Heravi, M. M., & TavakoliHoseini, N. (2012). Sulfonated carbon catalyzed Biginelli reaction for one-pot synthesis of 3,4-dihydropyrimidin-2(1H)ones and -thiones. Chinese Journal of Catalysis, 33, 706–710. DOI: 10.1016/s1872–2067(11)60377-x.
Mohammadi, A. A., Mivechi, M., & Kefayati, H. (2008). Potassium aluminum sulfate (alum): an efficient catalyst for the one-pot synthesis of trisubstituted imidazoles. Monatshefte fur Chemie Chemical Monthly, 139, 935–937. DOI: 10.1007/s00706-008-0875-7.
Mohammed, A. F., Kokare, N. D., Sangshetti, J. N., & Shinde, D. B. (2007). Sulphanilic acid catalyzed facile one-pot synthesis of 2,4,5-triarylimidazoles from benzil/benzoin and aromatic aldehydes. Journal of the Korean Chemical Society, 51, 418–422. DOI: 10.5012/jkcs.2007.51.5.418.
Nemati, F., Heravi, M. M., & Saeedi Rad, R. (2012). NanoFe3O4 encapsulated-silica particles bearing sulfonic acid groups as a magnetically separable catalyst for highly efficient Knoevenagel condensation and Michael addition reactions of aromatic aldehydes with 1,3-cyclic diketones. Chinese Journal of Catalysis, 33, 1825–1831. DOI: 10.1016/s1872-2067(11)60455-5.
Nouri Sefat, M., Deris, A., & Niknam, K. (2011). Preparation of silica-bonded propyl-diethylene-triamine-N-sulfamic acid as a recyclable catalyst for chemoselective synthesis of 1,1diacetates. Chinese Journal of Chemistry, 29, 2361–2367. DOI: 10.1002/cjoc.201180403.
Oskooie, H. A., Alimohammadi, Z., & Heravi, M. M. (2006a). Microwave-assisted solid-phase synthesis of 2,4,5-triaryl imidazoles in solventless system: An improved protocol. Heteroatom Chemistry, 17, 699–702. DOI: 10.1002/hc.20237.
Oskooie, H. A., Heravi, M. M., Bakhtiari, K., Zadsirjan, V., & Bamoharram, F. F. (2006b). H14[NaP5W30O110] as an efficient catalyst for the one-pot synthesis of a-amino nitriles. Synlett, 2006, 1768–1770. DOI: 10.1055/s-2006-944206.
Sadeghi, B., Mirjalili, B. B. F., & Hashemi, M. M. (2008). BF3-SiO2: an efficient reagent system for the one-pot synthesis of 1,2,4,5-tetrasubstituted imidazoles. Tetrahedron Letters, 49, 2575–2577. DOI: 10.1016/j.tetlet.2008.02.100.
Samai, S., Nandi, G. C., Singh, P., & Singh, M. S. (2009). L-Proline: an efficient catalyst for the one-pot synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles. Tetrahedron, 65, 10155–10161. DOI: 10.1016/j.tet.2009.10.019.
Sarshar, S., Siev, D., & Mjalli, A. M. M. (1996). Imidazole libraries on solid support. Tetrahedron Letters, 37, 835–838. DOI: 10.1016/0040–4039(95)02334–8.
Schaubroeck, D., De Baets, J., Desmet, T., Van Vlierberghe, S., Schacht, E., & Van Calster, A. (2009). Introduction of amino groups on the surface of thin photo definable epoxy resin layers via chemical modification. Applied Surface Science, 255, 8780–8787. DOI: 10.1016/j.apsusc.2009.06.043.
Schaubroeck, D., De Baets, J., Desmet, T., Dubruel, P., Schacht, E., Van Vaeck, L., & Van Calster, A. (2010). Surface modification of an epoxy resin with polyamines via cyanuric chloride coupling. Applied Surface Science, 256, 6269–6278. DOI: 10.1016/j.apsusc.2010.04.003.
Schmidt, M. W., Baldridge, K. K., Boatz, J. A., Elbert, S. T., Gordon, M. S., Jensen, J. H., Koseki, S., Matsunaga, N., Nguyen, K. A., Su, S. J., Windus, T. L., Dupuis, M., & Montgomery, J. A., Jr. (1993). General atomic and molecular electronic structure system. Journal of Computational Chemistry, 14, 1347–1363. DOI: 10.1002/jcc.540141112.
Shaabani, A., & Rahmati, A. (2006). Silica sulfuric acid as an efficient and recoverable catalyst for the synthesis of trisubstituted imidazoles. Journal of Molecular Catalysis A: Chemical, 249, 246–248. DOI: 10.1016/j.molcata.2006.01.006.
Sharma, S. D., Hazarika, P., & Konwar, D. (2008). An efficient and one-pot synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles catalyzed by InCl3⊙3H2O. Tetrahedron Letters, 49, 2216–2220. DOI: 10.1016/j.tetlet.2008.02.053.
Sharma, R. K., & Sharma, C. (2011). Zirconium(IV)-modified silica gel: Preparation, characterization and catalytic activity in the synthesis of some biologically important molecules. Catalysis Communications, 12, 327–331. DOI: 10.1016/j.catcom.2010.10.011.
Shvo, Y., & Goldman-Lev, V. (2002). Catalytic oxidation of alcohols with allyl diethyl phosphate and palladium acetate. Journal of Organometallic Chemistry, 650, 151–156. DOI: 10.1016/s0022–328x(02)01177–4.
Siddiqui, S. A., Narkhede, U. C., Palimkar, S. S., Daniel, T., Lahoti, R. J., & Srinivasan, K. V. (2005). Room temperature ionic liquid promoted improved and rapid synthesis of 2,4,5-triaryl imidazoles from aryl aldehydes and 1,2-diketones or α-hydroxyketone. Tetrahedron, 61, 3539–3546. DOI: 10.1016/j.tet.2005.01.116.
Silva, A. R., Freire, C., de Castro, B., Freitas, M. M. A., & Figueiredo, J. L. (2001). Anchoring of a nickel(II) Schiff base complex onto activated carbon mediated by cyanuric chloride. Microporous and Mesoporous Materials, 46, 211–221. DOI: 10.1016/s1387-1811(01)00297-9.
Silva, A. R., Wilson, K., Clark, J. H., & Freire, C. (2006). Covalent attachment of chiral manganese(III) salen complexes onto functionalised hexagonal mesoporous silica and application to the asymmetric epoxidation of alkenes. Microporous and Mesoporous Materials, 91, 128–138. DOI: 10.1016/j.micromeso.2005.11.032.
Silva, A. R., Budarin, V., Clark, J. H., Freire, C., & de Castro, B. (2007). Organo-functionalized activated carbons as supports for the covalent attachment of a chiral manganese(III) salen complex. Carbon, 45, 1951–1964. DOI: 10.1016/j.carbon.2007.06.013.
Suib, S. L. (2013). New and future developments in catalysis. Amsterdam, The Netherlands: Elsevier. DOI: 10.1016/b9780-444-53874-1.00022-6.
Tao, F., Nguyen, L., & Zhang, S. (2014). Introduction: Synthesis and catalysis on metal nanoparticles. In F. Tao (Ed.), Metal nanoparticles for catalysis: Advances and applications (RSC Catalysis Series, pp. 1–5). Cambridge, UK: The Royal Society of Chemistry. DOI: 10.1039/9781782621034-00001.
Wang, X. B., He, L., Jian, T. Y., & Ye, S. (2012). Cyclic phosphoric acid catalyzed one-pot, four-component synthesis of 1,2,4,5-tetrasubstituted imidazoles. Chinese Chemical Letters, 23, 13–16. DOI: 10.1016/j.cclet.2011.09.018.
Zeinali-Dastmalbaf, M., Davoodnia, A., Heravi, M. M., Tavakoli-Hoseini, N., Khojastehnezhad, A., & Zamani, H. A. (2011). Silica gel-supported polyphosphoric acid (PPA-SiO2) catalyzed one-pot multi-component synthesis of 3,4-dihydropyrimidin-2(1H)-ones and -thiones: An efficient method for the Biginelli reaction. Bulletin of Korean Chemical Society, 32, 656–658. DOI: 10.5012/bkcs.2011.32.2.656.
Zhao, Y., & Truhlar, D. G. (2008). The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theoretical ChemistryAccounts, 120, 215–241. DOI: 10.1007/s00214007-0310-x.
Zhao, X. N., Hu, H. C., Zhang, F. J., & Zhang, Z. H. (2014). Magnetic CoFe2O4 nanoparticle immobilized N-propyl diethylenetriamine sulfamic acid as an efficient and recyclable catalyst for the synthesis of amides via the Ritter reaction. Applied Catalysis A: General, 482, 258–265. DOI: 10.1016/j.apcata.2014.06.006.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mirsafaei, R., Heravi, M.M., Ahmadi, S. et al. Synthesis and properties of novel reusable nano-ordered KIT-5-N-sulfamic acid as a heterogeneous catalyst for solvent-free synthesis of 2,4,5-triaryl-1 H-imidazoles. Chem. Pap. 70, 418–429 (2016). https://doi.org/10.1515/chempap-2015-0228
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/chempap-2015-0228