Abstract
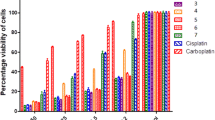
Cisplatin is an anticancer drug widely used in the treatment of a wide range of solid tumours (head and neck, lung, bladder etc.), testicular and ovarian cancers. Because of its severe toxicity profile and spontaneous development of drug resistance in tumours, a number of Pt(II) complexes have been synthesised and tested for anti-tumour activity. Some of the investigations have focused on using ligands bearing donor atoms other than N (e.g., S, P, O). Two new mixed Pt(II) complexes of the general formula cis-[Pt(NH3)LCl2] where L is 3-thiolanespiro-5′-hydantoin and 4-thio-1H-tetrahydropyranspiro-5′-hydantom were synthesised. The complexes were studied by elemental analysis, melting points, IR and 1H NMR spectra. The hybrid DFT calculations were used for optimisation of the structure geometries of the ligands III, IV and their Pt(II) complexes V and VI. The structural parameters so calculated, such as bond lengths and angles, are in good agreement with the experimental data for similar hydantoins and their platinum complexes. The results showed that the geometries of complexes V and VI are plane square and the bounding of ligands III and IV with platinum ions is effected by the sulphur atom from the cyclic ring. The complexes thus obtained were chemically examined in comparison with previously synthesised and published complexes of the general formula cis-[PtL2Cl2] (VII and VIII) with the same ligands. The new compounds V and VI, as well as the previously investigated complexes (VII and VIII), were analysed for cytotoxicity in vitro on SKW-3 and HL-60 human tumour cell lines. The results showed that all the complexes exerted concentration-dependent anti-proliferative activity.
Similar content being viewed by others
References
Abdulrahman, L. K., Al-Mously, M. M., Al-Mosuli, M. L., & Al-Azzawii, K. K. (2013). The biological activity of 5,5′-imidazolodine-2,4-dione derivatives. International Journal of Pharmacy and Pharmaceutical Sciences, 5, 494–504.
Abu-Surrah, A. S., & Kettunen, M. (2006). Platinum group antitumor chemistry: Design and development of new anti-cancer drugs complementary to cisplatin. Current Medicinal Chemistry, 13, 1337–1357. DOI: 10.2174/092986706776872970.
Bakalova, A., Varbanov, H., Buyukliev, R., Stanchev, S., Momekov, G., & Ivanov, D. (2010). Novel Pt(II) and Pt(IV) complexes with 3-amino-5-methyl-5-(4-pyridyl)-2,4-imidazolidenedione. Synthesis, physicochemical, chemometric and pharmacological investigation. Inorganica Chimica Acta, 363, 1568–1576. DOI: 10.1016/j.ica.2010.01.008.
Bakalova, A., Buyukliev, R., Momekov, G., & Ivanov, D. (2013). Synthesis and cytotoxic activity of new platinum and palladium complexes with 3-amino-α-tetralonespiro-5′-hydantoin. Journal of Chemical Technology and Metallurgy, 48, 631–636.
Bakalova, A., Buyukliev, R., & Momekov, G. (2015). Synthesis, DFT calculations and cytotoxic investigation of platinum complexes with 3-thiolanespiro-5′-hydantoin and 4-thio-1H-tetrahydropyranespiro-5′-hydantoin. Journal of Molecular Structure, 1091, 118–124. DOI: 10.1016/j.molstruc.2015.02.055.
Bierbach, U., Qu, Y., Hambley, T. W., Peroutka, J., Nguyen, H. L., Doedee, M., & Farell, N. (1999). Synthesis, structure, biological activity and DNA binding of platinum(II) complexes of the type trans-[PtCl2(NH3)L] (L = planar nitrogen base). Effect of L and cis/trans isomerism on sequence specificity and unwinding properties observed in globally platinated DNA. Inorganic Chemistry, 38, 3535–3542. DOI: 10.1021/ic.981181x.
Bogdanović, G., Kojić, V., Srdić, T., Jakimov, D., Djuran, M. I., Bugarčić, Ž. D., Baltić, M., & Baltić, V. V. (2002). Growth effects of some platinum(II) complexes with sulphur-containing carrier ligands on MCF7 human breast cancer cell line upon simultaneous administration with taxol. Metal-Based Drugs, 9, 33–43. DOI: 10.1155/mbd.2002.33.
Bugarčić, Ž. D., Bogojeski, J., Petrović, B., Hochreuther, S., & van Eldik, R. (2012). Mechanistic studies on the reactions of platinum(II) complexes with nitrogen and sulphur-donor biomolecules. Dalton Transactions, 41, 12329–12345. DOI: 10.1039/c2dt31045g.
Dylag, D., Zygmunt, M., Maciag, D., Handzlik, D., Bednarski, M., Filipek, B., & Kieć-Kinonowicz, K. (2004). Synthesis and evaluation of in vivo activity of diphenylhydantoin basic derivatives. European Journal of Medicinal Chemistry, 39, 1013–1027. DOI: 10.1016/j.ejmech.2004.05.008.
Friebolin, W., Schilling, G., Zöller, M., & Amtmann, E. (2004). Synthesis and structure-activity relationship of novel anti-tumoral platinum xanthate complexes. Journal of Medicinal Chemistry, 47, 2256–2263. DOI: 10.1021/jm0309405.
Frisch, M. J., Trucks, G., Schlegel, H., Scuseria, G., Robb, M., Cheeseman, J., Scalmani, G., Barone, V., Mennucci, B., Petersson, G., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H., Izmaylov, A., Bloino, J., Zheng, G., Sonnenberg, J., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J., Peralta, J., Ogliaro, F., Bearpark, M., Heyd, J., Brothers, E., Kudin, K., Staroverov, V., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J., Iyengar, S., Tomasi, J., Cossi, M., Rega, N., Millam, J., Klene, M., Knox, J., Cross, J., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R., Yazyev, O., Austin, A., Cammi, R., Pomelli, C., Ochterski, J., Martin, R., Morokuma, K., Zakrzewski, V., Voth, G., Salvador, P., Dannenberg, J., Dapprich, S., Daniels, A., Farkas, O., Foresman, J., Ortiz, J., Cioslowski, J., & Fox, D. (2009). Gaussian 09, Revision A1, [computer software]. Wallingford, CT, USA: Gaussian.
Ghani, N. T. A., & Mansour, A. M. (2011). Structural and in vitro cytotoxicity studies on 1H-benzimidazol-2-ylmethyl-N-phenyl amine and its Pd(II) and Pt(II) complexes. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 81, 529–543. DOI: 10.1016/j.saa.2011.06.046.
Kavitha, C. V., Nambiar, M., Kumar, C. S. A., Choudhary, B., Muniyappa, K., Rangappa, K., & Raghavan, S. C. (2009). Novel derivatives of spirohydantoin induce growth inhibition followed by apoptosis in leukemia cells. Biochemical Pharmacology, 77, 348–363. DOI: 10.1016/j.bcp.2008.10.018.
Konstantinov, S. M., Eibl, H., & Berger, M. R. (1999). BCR-ABL influences the antileukaemic efficacy of alkylphosphocholines. British Journal of Haematology, 107, 365–374. DOI: 10.1046/j.1365-2141.1999.01700.x.
Kruger, G., Mdluli, P. S., Power, T. D., Raasch, T., & Singh, A. (2006). Experimental and computational studies of the regioselective protection of hydantoins using anhydride. Journal of Molecular Structure, 771, 165–170. DOI: 10.1016/j.theochem.2006.03.037.
Lee, C. T., Yang, W. T., & Parr, R. G. (1988). Development of the Cole-Salvetti correlation-energy formula into a functional of the electron density. Physical Review B, 37, 785–789. DOI: 10.1103/physrevb.37.785.
Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunology Methods, 65, 55–63. DOI: 10.1016/0022-1759(83)90303-4.
Nakamoto, K. (1978). Infrared and Raman spectra of inorganic and coordination compounds (3rd ed.). New York, NY, USA: Wiley.
Rosenberg, B., Vancamp, L., Trosko, J. E., & Mansour, V. H. (1969). Platinum compounds: a new class of potent antitumor agents. Nature, 222, 385–386. DOI: 10.1038/222385a0.
Stephens, P. J., Devlin, F. J., Chabalowski, C. F., & Frisch, M. J. (1994). Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. The Journal of Physical Chemistry, 98, 11623–11627. DOI: 10.1021/j100096a001.
Thenmozhiyal, J. C., Wong, P. T. H., & Chui, W. K. (2004). Anticonvulsant activity of phenylmethylenehydantoins: A structure-activity relationship study. Journal of Medicinal Chemistry, 47, 1527–1535. DOI: 10.1021/jm030450c.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bakalova, A., Nikolova-Mladenova, B., Buyukliev, R. et al. Synthesis, DFT calculations and characterisation of new mixed Pt(II) complexes with 3-thiolanespiro-5′-hydantoin and 4-thio-1H-tetrahydropyranspiro-5′-hydantoin. Chem. Pap. 70, 93–100 (2016). https://doi.org/10.1515/chempap-2015-0194
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/chempap-2015-0194