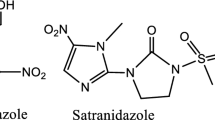
Abstract
Nanorod vanadatesulfuric acid (VSA NRs), as a recyclable and eco-benign catalyst, was used for one-pot synthesis of 2,4,5-trisubstituted imidazoles and 1,2,4,5-tetrasubstituted imidazoles using aldehydes, benzil, benzoin or 9,10-phenanthrenequinone and ammonium acetate or aniline under solvent-free conditions providing high to excellent yields. VSA is easily prepared by a simple reaction of chlorosulfonic acid and sodium metavanadate in high purity. As compared with the conventional procedures, the present protocol offers several advantages such as simplicity of procedure, short reaction time, high yields, easy workup, recoverability and reusability of the catalyst and simple purification of the products.
Similar content being viewed by others
References
Abrahams, S. L., Hazen, R. J., Batson, A. G., & Phillips, A. P. (1989). Trifenagrel: A chemically novel platelet aggregation inhibitor. Journal of Pharmacology and Experimental Therapeutics, 249 359–365.
Antolini, M., Bozzoli, A., Ghiron, C., Kennedy, G., Rossi, T., & Ursini, A. (1999). Analogues of 4,5-bis(3,5-dichlorophenyl)-2-trifluoromethyl-1H-imidazole as potential antibacterial agents. Bioorganic & Medicinal Chemistry Letters, 9 1023–1028. DOI: 10.1016/s0960-894x(99)00112-2.
Balalaie, S., Hashemi, M. M., & Akhbari, M. (2003). A novel one-pot synthesis of tetrasubstituted imidazoles under solvent-free conditions and microwave irradiation. Tetrahedron Letters, 44 1709–1711. DOI: 10.1016/s0040-4039(03)00018-2.
Bourissou, D., Guerret, O., Gabbaï, F. P., & Bertrand, G. (2000). Stable carbenes. Chemical Reviews, 100 39–92. DOI: 10.1021/cr940472u.
Chang, L. L., Sidler, K. L., Cascieri, M. A., de Laszlo, S., Koch, G., Li, B., MacCoss, M., Mantlo, N., O’Keefe, S., Pang, M., Rolando, A., & Hagmann, W. K. (2001). Substituted imidazoles as glucagon receptor antagonists. Bioorganic & Medicinal Chemistry Letters, 11 2549–2553. DOI: 10.1016/s0960-894x(01)00498-x.
Clark, J. H. (2002). Solid acids for green chemistry. Accounts of Chemical Research, 35 791–797. DOI: 10.1021/ar010072a.
Damavandi, S. (2011). Schiff base transition metal complex catalyzed one-pot synthesis of 2-aryl-1H-phenanthro[9,10-d]imidazoles. Synthesis and Reactivity in Inorganic, Metal-Organic and Nano-Metal Chemistry, 41 1274–1277. DOI: 10.1080/15533174.2011.594839.
Davidson, D., Weiss, M., & Jelling, M. (1937). The action of ammonia on benzil. The Journal of Organic Chemistry, 2 319–327. DOI: 10.1021/jo01227a004.
Davoodnia, A., Khojastehnezhad, A., Bakavoli, M., & Tavakoli-Hoseini, N. (2011). SO3H-functionalized ionic liquids: Green, efficient and reusable catalysts for the facile dehydration of aldoximes into nitriles. Chinese Journal of Chemistry, 29 978–982. DOI: 10.1002/cjoc.201190199.
Eseola, A. O., Li, W., Sun, W. H., Zhang, M., Xiao, L. W., & Woods, J. A. O. (2011). Luminescent properties of some imidazole and oxazole based heterocycles: Synthesis, structure and substituent effects. Dyes and Pigments, 88 262–273. DOI: 10.1016/j.dyepig.2010.07.005.
Frantz, D. E., Morency, L., Soheili, A., Murry, J. A., Grabowski, E. J. J., & Tillyer, R. D. (2004). Synthesis of substituted imidazoles via organocatalysis. Organic Letters, 6 843–846. DOI: 10.1021/ol0498803.
Heravi, M. M., Derikvand, F., & Bamoharram, F. F. (2007). Highly efficient, four-component one-pot synthesis of tetrasubstituted imidazoles using Keggin-type heteropolyacids as green and reusable catalysts. Journal of Molecular Catalysis A, 263 112–114. DOI: 10.1016/j.molcata.2006.08.048.
Karimi, A. R., Alimohammadi, Z., Azizian, J., Mohammadi, A. A., & Mohammadizadeh, M. R. (2006). Solvent-free synthesis of tetrasubstituted imidazoles on silica gel/NaHSO4 support. Catalysis Communications, 7 728–732. DOI: 10.1016/j.catcom.2006.04.004.
Karimi, A. R., Alimohammadi, Z., & Amini, M. M. (2010). Wells-Dawson heteropolyacid supported on silica: A highly efficient catalyst for synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles. Molecular Diversity, 14 635–641. DOI: 10.1007/s11030-009-9197-x.
Kidwai, M., Mothsra, P., Bansal, V., & Goyal, R. (2006). Efficient elemental iodine catalyzed one-pot synthesis of 2,4,5-triarylimidazoles. Monatshefte für Chemie — Chemical Monthly, 137 1189–1194. DOI: 10.1007/s00706-006-0518-9.
Kidwai, M., Mothsra, P., Bansal, V., Somvanshi, R. K., Ethayathulla, A. S., Dey, S., & Singh, T. P. (2007). One-pot synthesis of highly substituted imidazoles using molecular iodine: A versatile catalyst. Journal of Molecular Catalysis A, 265 177–182. DOI: 10.1016/j.molcata.2006.10.009.
Kokare, N. D., Sangshetti, J. N., & Shinde, D. B. (2007). One-pot efficient synthesis of 2-aryl-1-arylmethyl-1H-benzimidazoles and 2,4,5-triaryl-1H-imidazoles using oxalic acid catalyst. Synthesis, 2007 2829–2834. DOI: 10.1055/s-2007-983872.
Lantos, I., Zhang, W. Y., Shui, X. Q., & Eggleston, D. S. (1993). Synthesis of imidazoles via hetero-Cope rearrangements. The Journal of Organic Chemistry, 58 7092–7095. DOI: 10.1021/jo00077a033.
Liu, J. P., Chen, J. B., Zhao, J. F., Zhao, Y. H., Li, L., & Zhang, H. B. (2003). A modified procedure for the synthesis of 1-arylimidazoles. Synthesis, 2003 2661–2666. DOI: 10.1055/s-2003-42444.
Lombardino, J. G., & Wiseman, E. H. (1974). Preparation and antiinflammatory activity of some nonacidic trisubstituted imidazoles. Journal of Medicinal Chemistry, 17 1182–1188. DOI: 10.1021/jm00257a011.
Nagarapu, L., Apuri, S., & Kantevari, S. (2007). Potassium dodecatugstocobaltate trihydrate (K5CoW12O40 · 3H2O): A mild and efficient reusable catalyst for the one-pot synthesis of 1,2,4,5-tetrasubstituted imidazoles under conventional heating and microwave irradiation. Journal of Molecular Catalysis A, 266 104–108. DOI: 10.1016/j.molcata.2006.10.056.
Nasr-Esfahani, M., & Abdizadeh, T. (2013a). Nanorod vanadatesulfuric acid as a novel, recyclable and heterogeneous catalyst for the one-pot synthesis of tetrahydrobenzopyrans. Journal of Nanoscience and Nanotechnology, 13 5004–5011. DOI: 10.1166/jnn.2013.7592.
Nasr-Esfahani, M., & Abdizadeh, T. (2013b). Preparation, characterization and use of vanadatesulfuric acid as a new and eco-benign nanocatalyst for the synthesis of 14-aryl-14H-dibenzo[a,j] xanthenes under solvent-free conditions. Revue Roumaine de Chimie, 58 27–35.
Nasr-Esfahani, M., Hoseini, S. J., Montazerozohori, M., Mehrabi, R., & Nasrabadi, H. (2014). Magnetic Fe3O4 nanoparticles: Efficient and recoverable nanocatalyst for the synthesis of polyhydroquinolines and Hantzsch 1,4-dihydropyridines under solvent-free conditions. Journal of Molecular Catalysis A, 382 99–105. DOI: 10.1016/j.molcata.2013.11.010.
Norwood, D., Branch, E., Smith, B., & Honeywell, M. (2002). Olmesartan medoxomil for hypertension: A clinical review. Drug Forecast, 27 611–618.
Oskooie, H. A., Alimohammadi, Z., & Heravi, M. M. (2006). Microwave-assisted solid-phase synthesis of 2,4,5-triaryl imidazoles in solventless system: An improved protocol. Heteroatom Chemistry, 17 699–702. DOI: 10.1002/hc.20237.
Radziszewski, B. (1882). Ueber die Constitution des Lophins und verwandter Verbindungen. Berichte der Deutschen Chemischen Gesellschaft, 15 1493–1496. DOI: 10.1002/cber.18820150207. (in German)
Sadeghi, B., Mirjalili, B. B. F., & Hashemi, M. M. (2008). BF3 · SiO2: An efficient reagent system for the one-pot synthesis of 1,2,4,5-tetrasubstituted imidazoles. Tetrahedron Letters, 49 2575–2577. DOI: 10.1016/j.tetlet.2008.02.100.
Saffari Jourshari, M., Mamaghani, M., Shirini, F., Tabatabaeian, K., Rassa, M., & Langari, H. (2013). An expedient one-pot synthesis of highly substituted imidazoles using supported ionic liquid-like phase (SILLP) as a green and efficient catalyst and evaluation of their anti-microbial activity. Chinese Chemical Letters, 24 993–996. DOI: 10.1016/j.cclet.2013.06.005.
Samai, S., Nandi, G. C., Singh, P., & Singh, M. S. (2009). l-Proline: An efficient catalyst for the one-pot synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles. Tetrahedron, 65 10155–10161. DOI: 10.1016/j.tet.2009.10.019.
Sangshetti, J. N., Kokare, N. D., Kotharkara, S. A., & Shinde, D. B. (2008). Ceric ammonium nitrate catalysed three component one-pot efficient synthesis of 2,4,5-triaryl-1H-imidazoles. Journal of Chemical Sciences, 120 463–467. DOI: 10.1007/s12039-008-0072-6.
Shaabani, A., & Rahmati, A. (2006). Silica sulfuric acid as an efficient and recoverable catalyst for the synthesis of trisubstituted imidazoles. Journal of Molecular Catalysis A, 249 246–248. DOI: 10.1016/j.molcata.2006.01.006.
Siddiqui, S. A., Narkhede, U. C., Palimkar, S. S., Daniel, T., Lahoti, R. J., & Srinivasan, K. V. (2005). Room temperature ionic liquid promoted improved and rapid synthesis of 2,4,5-triaryl imidazoles from aryl aldehydes and 1,2-diketones or α-hydroxyketone. Tetrahedron, 61 3539–3546. DOI: 10.1016/j.tet.2005.01.116.
Sivakumar, K., Kathirvel, A., & Lalitha, A. (2010). Simple and efficient method for the synthesis of highly substituted imidazoles using zeolite-supported reagents. Tetrahedron Letters, 51 3018–3021. DOI: 10.1016/j.tetlet.2010.04.013.
Stähelin, M., Burland, D. M., Ebert, M., Miller, R. D., Smith, B. A., Twieg, R. J., Volksen, W., & Walsh, C. A. (1992). Re-evaluation of the thermal stability of optically nonlinear polymeric guest-host systems. Applied Physics Letters, 61 1626–1628. DOI: 10.1063/1.108497.
Sun, Y. F., Huang, W., Lu, C. G., & Cui, Y. P. (2009). The synthesis, two-photon absorption and blue upconversion fluorescence of novel, nitrogen-containing heterocyclic chromophores. Dyes and Pigments, 81 10–17. DOI: 10.1016/j.dyepig.2008.08.003.
Synećek, V., & Hanic, F. (1954). The crystal structure of ammonium metavanadate. Czechoslovak Journal of Physics, 4 120–129. DOI: 10.1007/bf01687750.
Takle, A. K., Brown, M. J. B., Davies, S., Dean, D. K., Francis, G., Gaiba, A., Hird, A. W., King, F. D., Lovell, P. J., Naylor, A., Reith, A. D., Steadman, J. G., & Wilson, D. M. (2006). The identification of potent and selective imidazole-based inhibitors of B-Raf kinase. Bioorganic & Medicinal Chemistry Letters, 16 378–381. DOI: 10.1016/j.bmcl.2005.09.072.
Teimouri, A., & Chermahini, A. N. (2011). An efficient and one-pot synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles catalyzed via solid acid nanocatalyst. Journal of Molecular Catalysis A, 346 39–45. DOI: 10.1016/j.molcata.2011.06.007.
Uçucu, Ü., Karaburun, N. G., & Işikdağ, İ (2001). Synthesis and analgesic activity of some 1-benzyl-2-substituted-4,5-diphenyl-1H-imidazole derivatives. Il Farmaco, 56 285–290. DOI: 10.1016/s0014-827x(01)01076-x.
Wang, L., Woods, K. W., Li, Q., Barr, K. J., McCroskey, R. W., Hannick, S. M., Gherke, L., Credo, R. B., Hui, Y. H., Marsh, K., Warner, R., Lee, J. Y., Zielinsky-Mozng, N., Frost, D., Rosenberg, S. H., & Sham, H. L. (2002). Potent, orally active heterocycle-based combretastatin A-4 analogues: Synthesis, structure-activity relationship, pharmacokinetics and in vivo antitumor activity evaluation. Journal of Medicinal Chemistry, 45 1697–1711. DOI: 10.1021/jm010523x.
Wang, J., Mason, R., VanDerveer, D., Feng, K., & Bu, X. R. (2003). Convenient preparation of a novel class of imidazo[1,5-a]pyridines: Decisive role by ammonium acetate in chemoselectivity. The Journal of Organic Chemistry, 68 5415–5418. DOI: 10.1021/jo0342020.
Wang, L. M., Wang, Y. H., Tian, H., Yao, Y. F., Shao, J. H., & Liu, B. (2006). Ytterbium triflate as an efficient catalyst for one-pot synthesis of substituted imidazoles through three-component condensation of benzil, aldehydes and ammonium acetate. Journal of Fluorine Chemistry, 127 1570–1573. DOI: 10.1016/j.jfluchem.2006.08.005.
Wang, X. C., Gong, H. P., Quan, Z. J., Li, L., & Ye, H. L. (2009). PEG-400 as an efficient reaction medium for the synthesis of 2,4,5-triaryl-1H-imidazoles and 1,2,4,5-tetraaryl-1H-imidazoles. Chinese Chemical Letters, 20 44–47. DOI: 10.1016/j.cclet.2008.10.005.
Weinmann, H., Harre, M., Koeing, K., Merten, E., & Tilestam, U. (2002). Efficient and environmentally friendly synthesis of 2-amino-imidazole. Tetrahedron Letters, 43 593–595. DOI: 10.1016/s0040-4039(01)02226-2.
Wolkenberg, S. E., Wisnoski, D. D., Leister, W. H., Wang, Y., Zhao, Z. J., & Lindsley, C. W. (2004). Efficient synthesis of imidazoles from aldehydes and 1,2-diketones using microwave irrediation. Organic Letters, 6 1453–1456. DOI: 10.1021/ol049682b.
Yan, Y. N., Lin, D. Y., Pan, W. L., Li, X. L., Wan, Y. Q., Mai, Y. L., & Song, H. C. (2009). Synthesis and optical behaviors of 2-(9-phenanthrenyl)-, 2-(9-anthryl)- and 2-(1-pyrenyl)-1-alkylimidazole homologues. Spectrochimica Acta Part A, 74 233–242. DOI: 10.1016/j.saa.2009.06.020.
Yan, Y. N., Pan, W. L., & Song, H. C. (2010). The synthesis and optical properties of novel 1,3,4-oxadiazole derivatives containing an imidazole unit. Dyes and Pigments, 86 249–258. DOI: 10.1016/j.dyepig.2010.01.011.
Zang, H. J., Su, Q. H., Mo, Y. M., Cheng, B. W., & Jun, S. (2010). Ionic liquid [EMIM]OAc under ultrasonic irradiation towards the first synthesis of trisubstituted imidazoles. Ultrasonics Sonochemistry, 17 749–751. DOI: 10.1016/j.ultsonch.2010.01.015.
Zhao, N., Wang, Y. L., & Wang, J. Y. (2005). A rapid and convenient synthesis of derivatives of imidazoles under microwave irradiation. Journal of the Chinese Chemical Society, 52 535–538. DOI: 10.1002/jccs.200500078.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nasr-Esfahani, M., Montazerozohori, M. & Abdizadeh, T. Multi-component synthesis of highly substituted imidazoles catalyzed by nanorod vanadatesulfuric acid. Chem. Pap. 69, 1491–1499 (2015). https://doi.org/10.1515/chempap-2015-0156
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/chempap-2015-0156