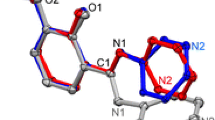
Abstract
One-pot anti-Mannich reaction of vanillin, aniline and cyclohexanone was successfully catalyzed by ionic liquid triethanolammonium chloroacetate, at room temperature. Yield of the obtained Mannich base was very good and excellent diastereoselectivity was achieved. Mechanism of the reaction was investigated using the density functional theory. The reaction started with a nucleophilic attack of aniline nitrogen at the carbonyl group of vanillin. The intermediate α-amino alcohol formed in this way was further subjected to protonation by the triethanolammonium ion yielding the imminium ion. Theoretically, the obtained imminium ion and the enol form of cyclohexanone can build the protonated Mannich base via the anti and syn pathways. The chloroacetic anion spontaneously abstracts the proton yielding the final product of the reaction anti 2-[1-(N-phenylamino)-1-(4-hydroxy-3-methoxyphenyl)]methylcyclohexanone (MB-H). The syn pathway requires lower activation energy but the anti pathway yields a thermodynamically more stable product, which implies that the examined Mannich reaction is thermodynamically controlled.
Similar content being viewed by others
References
Abbott, A. P., Capper, G., Davies, D. L., Rasheed, R. K., & Tambyrajah, V. (2002). Quaternary ammonium zinc- or tin-containing ionic liquids: Water insensitive, recyclable catalysts for Diels-Alder reactions. Green Chemistry, 4, 24–26. DOI: 10.1039/b108431c.
Aggarwal, A., Lancaster, N. L., Sethi, A. R., & Welton, T. (2002). The role of hydrogen bonding in controlling the selectivity of Diels-Alder reactions in room temperature ionic liquids. Green Chemistry, 4, 517–520. DOI: 10.1039/b206472c.
Akiyama, T., Takaya, J., & Kagoshima, H. (2001). A highly stereo-divergent Mannich-type reaction catalyzed by Brønsted acid in aqueous media. Tetrahedron Letters, 42, 4025–4028. DOI: 10.1016/s0040-4039(01)00648-7.
Arend, M., Westermann, B., & Risch, N. (1998). Modern variants of the Mannich reaction. Angewandte Chemie International Edition, 37, 1044–1070. DOI: 10.1002/(sici)1521-3773(19980504)37:8<1044∷aid-anie1044>3.0.co;2-e.
Azuma, T., Kobayashi, Y., Sakata, K., Sasamori, T., Tokitoh, N., & Takemoto, Y. (2014). Synthesis and characterization of binary-complex models of ureas and 1,3-dicarbonyl compounds: Deeper insights into reaction mechanisms using snap-shot structural analysis. The Journal of Organic Chemistry, 79, 1805–1817. DOI: 10.1021/jo4028775.
Barone, V., & Cossi, M. (1998). Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. Journal of Physical Chemistry A, 102, 1995–2001. DOI: 10.1021/jp9716997.
Chang, Q., Zhou, J., & Gan, L. H. (2012). Theoretical study on the mechanisms of proline-catalyzed Mannich reaction between acetaldehyde and N-Boc imines. Journal of Physical Organic Chemistry, 25, 667–673. DOI: 10.1002/poc.2898.
Chiappe, C., & Pieraccini, D. (2005). Ionic liquids: Solvent properties and organic reactivity. Journal of Physical Organic Chemistry, 18, 275–297. DOI: 10.1002/poc.863.
Cole, A. C., Jensen, J. L., Ntai, I., Tran, K. L. T., Weaver, K. J., Forbes, D. C., & Davis, J. H. (2002). Novel Brønsted acidic ionic liquids and their use as dual solvent — catalysts. Journal of the American Chemical Society, 124, 5962–5963. DOI: 10.1021/ja026290w.
Córdova, A. (2004). The direct catalytic asymmetric Mannich reaction. Accounts of Chemical Research, 37, 102–112. DOI: 10.1021/ar030231l.
Cossi, M., Rega, N., Scalmani, G., & Barone, V. (2003). Energies, structures and electronic properties of molecules in solution with the C-PCM solvation model. Journal of Computational Chemistry, 24, 669–681. DOI: 10.1002/jcc.10189.
Cota, I., Gonzalez-Olmos, R., Iglesias, M., & Medina, F. (2007). New short aliphatic chain ionic liquids: Synthesis, physical properties and catalytic activity in aldol condensations. The Journal of Physical Chemistry B, 111, 12468–12477. DOI: 10.1021/jp073963u.
Dong, F., Jun, L., Zhou, X. L., & Liu, Z. L. (2007). Mannich reaction in water using acidic ionic liquid as recoverable and reusable catalyst. Catalysis Letters, 116, 76–80. DOI: 10.1007/s10562-007-9095-8.
Fang, D., Gong, K., Zhang, D. Z., & Liu, Z. L. (2009). One-pot, three-component Mannich-type reaction catalyzed by functionalized ionic liquid. Monatshefte für Chemie — Chemical Monthly, 140, 1325–1329. DOI: 10.1007/s00706-009-0182-y.
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G. A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H. P., Izmaylov, A. F., Bloino, J., Zheng, G., Sonnenberg, J. L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J. A., Jr., Peralta, J. E., Ogliaro, F., Bearpark, M., Heyd, J. J., Brothers, E., Kudin, K. N., Staroverov, V. N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J. C., Iyengar, S. S., Tomasi, J., Cossi, M., Rega, N., Millam, J. M., Klene, M., Knox, J. E., Cross, J. B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J. W., Martin, R. L., Morokuma, K., Zakrzewski, V. G., Voth, G. A., Salvador, P., Dannenberg, J. J., Dapprich, S., Daniels, A. D., Farkas, O., Foresman, J. B., Ortiz, J. V., Cioslowski, J., & Fox, D. J. (2009). Gaussian 09 [computer software]. Wellingford, CT, USA: Gaussian Inc. Wallingford CT.
Fu, A. P., Li, H. L., Si, H. Z., Yuan, S. P., & Duan, Y. B. (2008). Theoretical studies of stereoselectivities in the direct syn- and anti-Mannich reactions catalyzed by different amino acids. Tetrahedron: Asymmetry, 19, 2285–2292. DOI: 10.1016/j.tetasy.2008.09.023.
Gong, K., Fang, D., Wang, H. L., & Liu, Z. L. (2007). Basic functionalized ionic liquid catalyzed one-pot Mannich-type reaction: Three component synthesis of β-amino carbonyl compounds. Monatshefte für Chemie — Chemical Monthly, 138, 1195–1198. DOI: 10.1007/s00706-007-0767-2.
Huddleston, J. G., Visser, A. E., Reichert, W. M., Willauer, H. D., Broker, G. A., & Rogers, R. D. (2001). Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chemistry, 3, 156–164. DOI: 10.1039/b103275p.
Ibrahem, I., Zou, W. B., Casas, J., Sundén, H., & Córdova, A. (2006). Direct organocatalytic enantioselective α-aminomethylation of ketones. Tetrahedron, 62, 357–364. DOI: 10.1016/j.tet.2005.08.113.
Iglesias, M., Gonzalez-Olmos, R., Cota, I., & Medina, F. (2010). Brønsted ionic liquids: Study of physico-chemical properties and catalytic activity in aldol condensations. Chemical Engineering Journal, 162, 802–808. DOI: 10.1016/j.cej.2010.06.008.
Janey, J. M., Hsiao, Y., & Armstrong, J. D. (2006). Proline-catalyzed, asymmetric Mannich reactions in the synthesis of a DPP-IV inhibitor. The Journal of Organic Chemistry, 71, 390–392. DOI: 10.1021/jo0519458.
Kantam, M. L., Rajasekhar, C. V., Gopikrishna, G., Rajender Reddy, K., & Choudary, B. M. (2006). Proline catalyzed two-component, three-component and self-asymmetric Mannich reactions promoted by ultrasonic conditions. Tetrahedron Letters, 47, 5965–5967. DOI: 10.1016/j.tetlet.2006.06.042.
Keskin, S., Kayrak-Talay, D., Akman, U., & Hortaçsu, Ö. (2007). A review of ionic liquids towards supercritical fluid applications. The Journal of Supercritical Fluids, 43, 150–180. DOI: 10.1016/j.supflu.2007.05.013.
Kumar, A., & Pawar, S. S. (2004). Converting exo-selective Diels-Alder reaction to endo-selective in chloroloaluminate ionic liquids. The Journal of Organic Chemistry, 69, 1419–1420. DOI: 10.1021/jo035038j.
List, B., Pojarliev, P., Biller, W. T., & Martin, H. J. (2002). The proline-catalyzed direct asymmetric three-component Mannich reaction: Scope, optimization and application to the highly enantioselective synthesis of 1,2-amino alcohols. Journal of the American Chemical Society, 124, 827–833. DOI: 10.1021/ja0174231.
Liu, B. Y., Xu, D. Q., Dong, J. F., Yang, H. L., Zhao, D. S., Luo, S. P., & Xu, Z. Y. (2007). Highly efficient AILs/l-proline synergistic catalyzed three-component asymmetric Mannich reaction. Synthetic Communications, 37, 3003–3010. DOI: 10.1080/00397910601163976.
Loh, T. P., Liung, S. B. K. W., Tan, K. L., & Wei, L. L. (2000). Three component synthesis of β-amino carbonyl compounds using indium trichloride-catalyzed one-pot Mannich-type reaction in water. Tetrahedron, 56, 3227–3237. DOI: 10.1016/s0040-4020(00)00221-0.
Loh, T. P., & Chen, S. L. (2002). InCl3-Catalyzed three-component asymmetric Mannich-type reaction in methanol. Organic Letters, 4, 3647–3650. DOI: 10.1021/ol0265968.
Manabe, K., Mori, Y., Kobayashi, S. (2001). Three-component carbon-carbon bond-forming reactions catalyzed by a Brønsted acid — surfactant-combined catalyst in water. Tetrahedron, 57, 2537–2544. DOI: 10.1016/s0040-4020(01)00081-3.
Mukhopadhyay, C., Datta, A., & Butcher, R. J. (2009). Highly efficient one-pot, three-component Mannich reaction catalysed by boric acid and glycerol in water with major “syn” diastereoselectivity. Tetrahedron Letters, 50, 4246–4250. DOI: 10.1016/j.tetlet.2009.04.135.
Parasuk, W., & Parasuk, V. (2008). Theoretical investigations on the stereoselectivity of the proline catalyzed Mannich reaction in DMSO. The Journal of Organic Chemistry, 73, 9388–9392. DOI: 10.1021/jo801872w.
Petrović Z. D., Simijonović, D., Petrović, V. P., & Marković, S. (2010). Diethanolamine and N,N-diethylethanolamine ionic liquids as precatalyst-precursors and reaction media in green Heck reaction protocol. Journal of Molecular Catalysis A, 327, 45–50. DOI: 10.1016/j.molcata.2010.05.010.
Petrović Z. D., Marković, S., Petrović, V. P., & Simijonović, D. (2012). Triethanolammonium acetate as a multifunctional ionic liquid in the palladium-catalyzed green Heck reaction. Journal of Molecular Modeling, 18, 433–440. DOI: 10.1007/s00894-011-1052-1.
Petrović V. P., Simijonović, D., Živanović, M. N., Košarić, J. V., Petrović, Z. D., Marković, S., & Marković, S. D. (2014). Vanillic Mannich bases: Synthesis and screening of biological activity. Mechanistic insight into the reaction with 4-chloroaniline. RSC Advances, 4, 24635–24644. DOI: 10.1039/c4ra03909b.
Phukan, P., Kataki, D., & Chakraborty, P. (2006). Direct synthesis of Cbz-protected β-amino ketones by iodine-catalyzed three-component condensation of aldehydes, ketones and benzyl carbamate. Tetrahedron Letters, 47, 5523–5525. DOI: 10.1016/j.tetlet.2006.05.136.
Sahoo, S., Joseph, T., & Halligudi, S. B. (2006). Mannich reaction in Brønsted acidic ionic liquid: A facile synthesis of β-amino carbonyl compounds. Journal of Molecular Catalysis A, 244, 179–182. DOI: 10.1016/j.molcata.2005.09.012.
Shariati, A., & Peters, C. J. (2005). High-pressure phase equilibria of systems with ionic liquids. The Journal of Supercritical Fluids, 34, 171–176. DOI: 10.1016/j.supflu.2004.11.011.
Shariati, A., Gutkowski, K., & Peters, C. J. (2005). Comparison of the phase behavior of some selected binary systems with ionic liquids. AIChE Journal, 51, 1532–1540. DOI: 10.1002/aic.10384.
Simijonović, D., Petrović, Z. D., & Petrović, V. P. (2013). Some physico-chemical properties of ethanolamine ionic liquids: Behavior in different solvents. Journal of Molecular Liquids, 179, 98–103. DOI: 10.1016/j.molliq.2012.12.020.
Singh, R., Sharma, M., Mamgain, R., & Rawat, D. S. (2008). Ionic liquids: A versatile medium for palladium-catalyzed reactions. Journal of the Brazilian Chemical Society, 19, 357–379. DOI: 10.1590/s0103-50532008000300002.
Touré, B. B., & Hall, D. G. (2009). Natural product synthesis using multicomponent reaction strategies. Chemical Reviews, 109, 4439–4486. DOI: 10.1021/cr800296p.
Wang, L. M., Han, J. W., Sheng, J., Tian, H., & Fan, Z. Z. (2005). Rare earth perfluorooctanoate [RE(PFO)3] catalyzed one-pot Mannich reaction: Three component synthesis of β-amino carbonyl compounds. Catalysis Communications, 6, 201–204. DOI: 10.1016/j.catcom.2004.12.009.
Xiao, Y., & Malhotra, S. V. (2005). Friedel-Crafts alkylation reactions in pyridinium-based ionic liquids. Journal of Molecular Catalysis A, 230, 129–133. DOI: 10.1016/j.molcata.2004.12.015.
Yalalov, D. A., Tsogoeva, S. B., Shubina, T. E., Martynova, I. M., & Clark, T. (2008). Evidence for an enol mechanism in a highly enantioselective Mannich-type reaction catalyzed by primary amine-thiourea. Angewandte Chemie International Edition, 47, 6624–6628. DOI: 10.1002/anie.200800849.
Yang, Y. Y., Shou, W. G., & Wang, Y. G. (2006). Synthesis of β-amino carbonyl compounds via a Zn(OTf)2-catalyzed cascade reaction of anilines with aromatic aldehydes and carbonyl compounds. Tetrahedron, 62, 10079–10086. DOI: 10.1016/j.tet.2006.08.063.
Yang, J. W., Stadler, M., & List, B. (2007). Proline-catalyzed Mannich reaction of aldehydes with N-Boc-imines. Angewandte Chemie International Edition, 46, 609–611. DOI: 10.1002/anie.200603188.
Yi, W. B., & Cai, C. (2006). Mannich-type reactions of aromatic aldehydes, anilines and methyl ketones in fluorous biphase systems created by rare earth(III) perfluorooctane sulfonates catalysts in fluorous media. Journal of Fluorine Chemistry, 127, 1515–1521. DOI: 10.1016/j.jfluchem.2006.07.009.
Yin, D. H., Li, C. Y., Tao, L., Yu, N. G., Hu, S., & Yin, D. L. (2006). Synthesis of diphenylmethane derivatives in Lewis acidic ionic liquids. Journal of Molecular Catalysis A, 245, 260–265. DOI: 10.1016/j.molcata.2005.10.010.
Yue, C. B., Yi, T. F., Zhu, C. B., & Liu, G. (2009). Mannich reaction catalyzed by a novel catalyst under solvent-free conditions. Journal of Industrial and Engineering Chemistry, 15, 653–656. DOI: 10.1016/j.jiec.2009.09.038.
Zhao, G. Z., Jiang, T., Gao, H. X., Han, B. X., Huang, J., & Sun, D. H. (2004). Mannich reaction using acidic ionic liquids as catalysts and solvents. Green Chemistry, 6, 75–77. DOI: 10.1039/b309700p.
Zhao, H., Xia, S. Q., & Ma, P. S. (2005). Use of ionic liquids as ‘green’ solvents for extractions. Journal of Chemical Technology & Biotechnology, 80, 1089–1096. DOI: 10.1002/jctb.1333.
Zhao, Y., Schultz, N. E., & Truhlar, D. G. (2006). Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics and noncovalent interactions. Journal of Chemical Theory and Computation, 2, 364–382. DOI: 10.1021/ct0502763.
Zhou, X., Liu, B., Luo, F., Zhang, W., & Song, H. (2011). Novel Brønsted-acidic ionic liquids based on benzothiazolium cations as catalysts for esterification reactions. Journal of the Serbian Chemical Society, 76, 1607–1615. DOI: 10.2298/jsc110102144z.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Petrović, V.P., Simijonović, D., Petrović, Z.D. et al. Formation of a vanillic Mannich base — theoretical study. Chem. Pap. 69, 1244–1252 (2015). https://doi.org/10.1515/chempap-2015-0123
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/chempap-2015-0123