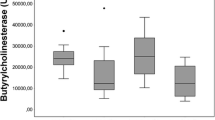
Abstract
Two cholinesterases: acetylcholinesterase (AChE) and butyrylcholinesterase (BChE), are known. The enzymes are important in the body and alteration of their activity has significant use in the diagnosis of poisoning, liver function, etc. Currently available methods for the determination of cholinesterases have some major drawbacks including various interferences and the inability to be used for decreasing the enzyme activity in the presence of reversible inhibitors due to sample dilution; hence, a method for dilution free assay of cholinesterases is desired. Here, microplates were modified with indoxylacetate (100 µL of 10 mmol L−1 solution) and used for cholinesterases assay after drying at 37°C. The fact that indoxylacetate remains stable in dry state and serves simultaneously as a chromogen and substrate provide good prerequisites for the method. The limit of detection for BChE was 0.71 U while that for AChE was 2.8 U per a 100 µL sample (solution of enzyme or plasma sample). The limit of detection is low enough to allow standard examination of cholinesterasemia. The two cholinesterases can be distinguished from each other using selective inhibitors such as donepezil and iso-OMPA. The new method was also successfully validated for the standard Ellman’s assay using plasma samples with BChE activity adjusted by carbofuran. The new method based on indoxylacetate seems promising for routine tests.
Similar content being viewed by others
References
Bazire, A., Gillon, E., Lockridge, O., Vallet, V., & Nachon, F. (2011). The kinetic study of the inhibition of human cholinesterases by demeton-S-methyl shows that cholinesterase-based titration methods are not suitable for this organophosphate. Toxicology in Vitro, 25, 754–759. DOI: 10.1016/j.tiv.2011.01.006.
Colović, M. B., Krstić, D. Z., Lazarević-Pašti, T. D., Bondzić, A. M., & Vasić, V. M. (2013). Acetylcholinesterase inhibitors: Pharmacology and toxicology. Current Neuropharmacology, 11, 315–335. DOI: 10.2174/1570159x11311030006.
Darreh-Shori, T., & Soininen, H. (2010). Effects of cholinesterase inhibitors on the activities and protein levels of cholinesterases in the cerebrospinal fluid of patients with Alzheimer’s disease: a review of recent clinical studies. Current Alzheimer Research, 7, 67–73. DOI: 10.2174/156720510790274455.
de Melo, J. S., Rondão, R., Burrows, H. D., Melo, M. J., Navaratnam, S., Edge, R., & Voss, G. (2006). Photophysics of an indigo derivative (keto and leuco structures) with singular properties. The Journal of Physical Chemistry A, 110, 13653–13661. DOI: 10.1021/jp057451w.
Duysen, E. G., & Lockridge, O. (2011). Prolonged toxic effects after cocaine challenge in butyrylcholinesterase/plasma carboxylesterase double knockout mice: a model for butyrylcholinesterase-deficient humans. Drug Metabolism & Disposition, 39, 1321–1323. DOI: 10.1124/dmd.111.039917.
Ellman, G. L., Courtney, K. D., Andres, V., Jr., & Featherstone, R. M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology, 7, 88–95. DOI: 10.1016/0006-2952(61)90145-9.
Eyer, P., Worek, F., Kiderlen, D., Sinko, G., Stuglin, A., Simeon-Rudolf, V., & Reiner, E. (2003). Molar absorption coefficients for the reduced Ellman reagent: reassessment. Analytical Biochemistry, 312, 224–227. DOI: 10.1016/s0003-2697(02)00506-7.
George, P. M., & Abernethy, M. H. (1983). Improved Ellman procedure for erythrocyte cholinesterase. Clinical Chemistry, 29, 365–368.
GhattyVenkataKrishna, P. K., Chavali, N., & Uberbacher, E. C. (2013). Flexibility of active-site gorge aromatic residues and non-gorge aromatic residues in acetylcholinesterase. Chemical Papers, 67, 677–681. DOI: 10.2478/s11696-013-0354-4.
Giustarini, D., Dalle-Donne, I., Milzani, A., Fanti, P., & Rossi, R. (2013). Analysis of GSH and GSSG after derivatization with N-ethylmaleimide. Nature Protocols, 8, 1660–1669. DOI: 10.1038/nprot.2013.095.
Gorun, V., Proinov, I., Băltescu, V., Balaban, G., & Bârzu, O. (1978). Modified Ellman procedure for assay of cholinesterases in crude enzymatic preparations. Analytical Biochemistry, 86, 324–326. DOI: 10.1016/0003-2697(78)90350-0.
Guemei, A. A., Cottrell, J., Band, R., Hehman, H., Prudhomme, M., Pavlov, M. V., Grem, J. L., Ismail, A. S., Bowen, D., Taylor, R. E., & Takimoto, C. H. (2001). Human plasma carboxylesterase and butyrylcholinesterase enzyme activity: correlations with SN-38 pharmacokinetics during a prolonged infusion of irinotecan. Cancer Chemotherapy and Pharmacology, 47, 283–290. DOI: 10.1007/s002800000258.
Harel, M., Sussman, J. L., Krejci, E., Bon, S., Chanal, P., Massoulié, J., & Silman, I. (1992). Conversion of acetylcholinesterase to butyrylcholinesterase: Modeling and mutagenesis. Proceedings of the National Academy of Sciences of the United States of America, 89, 10827–10831. DOI: 10.1073/pnas.89.22.10827.
Iwasaki, T., Yoneda, M., Nakajima, A., & Terauchi, Y. (2007). Serum butyrylcholinesterase is strongly associated with adiposity, the serum lipid profile and insulin resistance. Internal Medicine, 46, 1633–1639. DOI: 10.2169/internalmedicine.46.0049.
Kemkes-Matthes, B., Preissner, K. T., Langenscheidt, F., Matthes, K. J., & Müller-Berghaus, G. (1987). S protein/vitronectin in chronic liver diseases: correlations with serum cholinesterase, coagulation factor X and complement component C3. European Journal of Haematology, 39, 161–165. DOI: 10.1111/j.1600-0609.1987.tb00747.x.
Khaled, E., Hassan, H. N. A., Mohamed, G. G., Ragab, F. A., & Seleim, A. E. A. (2010). Disposable potentiometric sensors for monitoring cholinesterase activity. Talanta, 83, 357–363. DOI: 10.1016/j.talanta.2010.09.020.
Lejus, C., Delaroche, O., Trille, E., Blanloeil, Y., & Pinaud, M. (2006). Butyrylcholinesterase deficiency: how to analyse the cholinesterase activity in small children? Annales Françaises d’Anesthésie et de Réanimation, 25, 657–660. DOI: 10.1016/j.annfar.2006.02.009.
Pohanka, M. (2011). Cholinesterases, a target of pharmacology and toxicology. Biomedical Papers Olomouc, 155, 219–229. DOI: 10.5507/bp.2011.036.
Pohanka, M. (2012a). Acetylcholinesterase inhibitors: a patent review (2008 — present). Expert Opinion on Therapeutic Patents, 22, 871–886. DOI: 10.1517/13543776.2012.701620.
Pohanka, M. (2012b). Acetylcholinesterase based dipsticks with indoxylacetate as a substrate for assay of organophosphates and carbamates. Analytical Letters, 45, 367–374. DOI: 10.1080/00032719.2011.644743.
Pohanka, M. (2013a). Cholinesterases in biorecognition and biosensor construction: A review. Analytical Letters, 46, 1849–1868. DOI: 10.1080/00032719.2013.780240.
Pohanka, M. (2013b). Butyrylcholinesterase as a biochemical marker, a review. Bratislava Medical Journal, 114, 726–734. DOI: 10.4149/bll_2013_153.
Pohanka, M. (2014). Voltammetric assay of butyrylcholinesterase in plasma samples and its comparison to the standard spectrophotometric test. Talanta, 119, 412–416. DOI: 10.1016/j.talanta.2013.11.045.
Pohanka, M. (2015). Biosensors containing acetylcholinesterase and butyrylcholinesterase as recognition tools for detection of various compounds. Chemical Papers, 69, 4–16. DOI: 10.2478/s11696-014-0542-x.
Prellwitz, W., Kapp, S., & Müller, D. (1976). Comparative methods for the determination of the activity of serumcholinesterases (acylcholin-acyl-hydrolase E.C. 3.1.1.8) and their diagnostical value. Journal of Clinical Chemistry and Clinical Biochemistry, 14, 93–97. DOI: 10.1515/cclm.1976.14.1-12.93.
Prokofieva, D. S., Jenkins, R. O., & Goncharov, N. V. (2012). Microplate biochemical determination of Russian VX: Influence of admixtures and avoidance of false negative results. Analytical Biochemistry, 424, 108–113. DOI: 10.1016/j.ab.2012.02.022.
Rastogi, S. K., Singh, V. K., Kesavachandran, C., Jyoti, Siddiqui, M. K. J., Mathur, N., & Bharti, R. S. (2008). Monitoring of plasma butyrylcholinesterase activity and hematological parameters in pesticide sprayers. Indian Journal of Occupational & Environmental Medicine, 12, 29–32. DOI: 10.4103/0019-5278.40813.
Sochocka, M., Zaczyńska, E., Leszek, J., Siemieniec, W., & Blach-Olszewska, Z. (2008). Effect of donepezil on innate antiviral immunity of human leukocytes. Journal of the Neurological Sciences, 273, 75–80. DOI: 10.1016/j.jns.2008.06.021.
Villatte, F., Bachman, T. T., Hussein, A. S., & Schmid, R. D. (2001). Acetylcholinesterase assay for rapid expression screening in liquid and solid media. Bio Techniques, 30, 81–86.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pohanka, M. Determination of acetylcholinesterase and butyrylcholinesterase activity without dilution of biological samples. Chem. Pap. 69, 1044–1049 (2015). https://doi.org/10.1515/chempap-2015-0117
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/chempap-2015-0117