Abstract
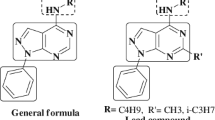
Progression of drug resistance among bacterial and fungal pathogens justifies the development of novel antimicrobial agents. Thus, a series of novel sulphamethoxazole-based ureas and imidazolidine-2,4,5-triones have been designed and synthesised. The urea derivatives were obtained by the reaction of sulphamethoxazole and isocyanates, and their cyclisation to imidazolidine-2,4,5-triones was performed via oxalyl chloride. All synthesised derivatives were evaluated in vitro to determine their activity against gram-positive and gram-negative bacteria, fungi, Mycobacterium tuberculosis, and atypical mycobacteria and their cytotoxicity. The growth of mycobacteria was inhibited within the range of 4–1000 µM and M. tuberculosis was the least-susceptible strain. 4-(3-Heptylureido)-N-(5-methylisoxazol-3-yl)benzenesulphonamide was identified as the most promising compound because it exhibited the highest activity against atypical mycobacteria at minimum inhibitory concentrations, from 4 µM, and with acceptable toxicity (selectivity indices for M. avium and M. kansasii higher than 16 and 62.5, respectively). Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus, were inhibited at concentrations starting from 125 µM, whereas the investigated derivatives exhibited almost no antifungal potency and activity against gram-negative species.
Similar content being viewed by others
References
Agertt, V. A., Marques, L. L., Bonez, P. C., Dalmolin, T. V., Manzoni de Oliveira, G. N., & Anraku de Campos, M. M. (2013). Evaluation of antimycobacterial activity of a sulphonamide derivative. Tuberculosis, 93, 318–321. DOI: 10.1016/j.tube.2013.02.003.
Akgün, H., Karamelekoğlu, I., Berk, B., Kurnaz, I., Sarıbıyık, G., Öktem, S., & Kocagöz, T. (2012). Synthesis and antimycobacterial activity of some phthalimide derivatives. Bioorganic & Medicinal Chemistry, 20, 4149–4154. DOI: 10.1016/j.bmc.2012.04.060.
Alsaad, N., van der Laan, T., van Altena, R., Wilting, K. R., van der Werf, T. S., Stienstra, Y., van Soolingen, D., & Alffenaar, J. W. C. (2013). Trimethoprim/sulfamethoxazole susceptibility of Mycobacterium tuberculosis. International Journal of Antimicrobial Agents, 42, 472–474. DOI: 10.1016/j.ijantimicag.2013.07.011.
Ameen, S. M., & Drancourt, M. (2013). In vitro susceptibility of Mycobacterium avium complex mycobacteria to trimethoprim and sulphonamides. International Journal of Antimicrobial Agents, 42, 281–282. DOI: 10.1016/j.ijantimicag.2013.05.006.
Brown, J. R., North, E. J., Hurdle, J. G., Morisseau, C., Scarborough, J. S., Sun, D., Korduláková, J., Scherman, M. S., Jones, V., Grzegorzewicz, A., Crew, R. M., Jackson, M., McNeil, M. R., & Lee, R. E. (2011). The structure-activity relationship of urea derivatives as anti-tuberculosis agents. Bioorganic & Medicinal Chemistry, 19, 5585–5595. DOI: 10.1016/j.bmc.2011.07.034.
Desai, S. R., Laddi, U., Bennur, R. S., Patil, P. A., & Bennur, S. (2011). Synthesis and pharmacological activities of some new 5-substituted-2-mercapto-1,3,4-oxadiazoles. Indian Journal of Pharmaceutical Sciences, 73, 593–596. DOI: 10.4103/0250-474x.99025.
Huang, T. S., Kunin, C. M., Yan, B. S., Chen, Y. S., Lee, S. S. J., & Syu, W. J. (2012). Susceptibility of Mycobacterium tuberculosis to sulfamethoxazole, trimethoprim and their combination over a 12 year period in Taiwan. Journal of Antimicrobial Chemotherapy, 67, 633–637. DOI: 10.1093/jac/dkr501.
Krátký, M., & Vinšová, J. (2012). Antifungal activity of salicylanilides and their esters with 4-(trifluoromethyl)benzoic acid. Molecules, 17, 9426–9442. DOI: 10.3390/molecules17089426.
Krátký, M., Vinšová, J., Volková, M., Buchta, V., Trejtnar, F., & Stolańíková, J. (2012). Antimicrobial activity of sulfonamides containing 5-chloro-2-hydroxybenzaldehyde and 5-chloro-2-hydroxybenzoic acid scaffold. European Journal of Medicinal Chemistry, 50, 433–440. DOI: 10.1016/j.ejmech.2012.01.060.
Krátký, M., Vinšová, J., Novotná, E., Mandíková, J., Trejtnar, F., & Stolańíková, J. (2013). Antibacterial activity of salicylanilide 4-(trifluoromethyl)benzoates. Molecules, 18, 3674–3688. DOI: 10.3390/molecules18043674.
Maresca, A., Scozzafava, A., Vullo, D., & Supuran, C. T. (2013). Dihalogenated sulfanilamides and benzolamides are effective inhibitors of the three β-class carbonic anhydrases from Mycobacterium tuberculosis. Journal of Enzyme Inhibition and Medicinal Chemistry, 28, 384–387. DOI: 10.3109/14756366.2011.645539.
Minakuchi, T., Nishimori, I., Vullo, D., Scozzafava, A., & Supuran, C. T. (2009). Molecular cloning, characterization, and inhibition studies of the Rv1284 β-carbonic anhydrase from Mycobacterium tuberculosis with sulfonamides and a sulfamate. Journal of Medicinal Chemistry, 52, 2226–2232. DOI: 10.1021/jm9000488.
North, E. J., Scherman, M. S., Bruhn, D. F., Scarborough, J. S., Maddox, M. M., Jones, V., Grzegorzewicz, A., Yang, L., Hess, T., Morisseau, C., Jackson, M., McNeil, M. R., & Lee, R. E. (2013). Design, synthesis and anti-tuberculosis activity of 1-adamantyl-3-heteroaryl ureas with improved in vitro pharmacokinetic properties. Bioorganic & Medicinal Chemistry, 21, 2587–2599. DOI: 10.1016/j.bmc.2013.02.028.
Pejchal, V., Stepankova, S., Padelkova, Z., Imramovsky, A., & Jampilek, J. (2011). 1,3-Substituted imidazolidine-2,4,5-triones: Synthesis and inhibition of cholinergic enzymes. Molecules, 16, 7565–7582. DOI: 10.3390/molecules16097565.
Sukdolak, S., Solujić, S., Manojlović, N., & Krstić, L. J. (2005). Synthesis and antimicrobial activity of new N-[4-(4-hydroxy-2-oxo-2H-chromen-3-yl)thiazol-2-yl]benzenesulfonamides. Chemical Papers, 59, 37–40.
Thomas, K. D., Adhikari, A. V., Chowdhury, I. H., Sumesh, E., & Pal, N. K. (2011). New quinolin-4-yl-1,2,3-triazoles carrying amides, sulphonamides and amidopiperazines as potential antitubercular agents. European Journal of Medicinal Chemistry, 46, 2503–2512. DOI: 10.1016/j.ejmech.2011.03.039.
Yun, M. K., Wu, Y., Li, Z., Zhao, Y., Waddell, M. B., Ferreira, A. M., Lee, R. E., Bashford, D., & White, S. W. (2012). Catalysis and sulfa drug resistance in dihydropteroate synthase. Science, 335, 1110–1114. DOI: 10.1126/science.1214641.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krátký, M., Mandíková, J., Trejtnar, F. et al. Synthesis and antimicrobial activity of sulphamethoxazole-based ureas and imidazolidine-2,4,5-triones. Chem. Pap. 69, 1108–1117 (2015). https://doi.org/10.1515/chempap-2015-0109
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/chempap-2015-0109