Abstract
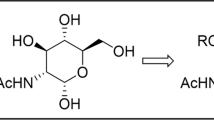
New potential transition state analogue inhibitors for N-acetylglucosyltransferases (GnTs) were synthesised. These compounds based on psico- and tagatofuranose (structure) scaffold contained a 2-thiophenyl-1-O-diethylphosphate moiety mimicking the proposed model of the transition state of the enzymatic reaction catalysed by N-acetylglucosyltransferases. The synthesised compounds as well as their precursors were fully characterised by NMR, optical rotation and mass techniques. Anomeric configuration of tagatofuranose derivatives was confirmed by X-ray crystallography. Two types of potential human glycosyltransferase (GnTs) inhibitors representing donor UDP-GlcNAc, assigned for biological assays on human GnTs, were prepared.
Similar content being viewed by others
References
Bachmann, W. E., Ross, A., Dreiding, A. S., & Smith, P. A. S. (1954) Relative stabilities of cis-trans isomers of fused ring systems containing angular methyl groups. Journal of Organic Chemistry, 19, 222–240. DOI: 10.1021/jo01367a010.
Baráth, M., Koóš, M., Tvaroška, I., & Hirsch, J. (2015) Synthesis of glycomimetics of the inhibitors of glycosyltransferases representing UDP-GlcNAc. Chemical Papers, 2015, in press. DOI: 10.1515/chempap-2015-0017.
Collins, E. S., Galligan, M. C., Saldova, R., Adamczyk, B., Abrahams, J. L., Campbell, M. P., Ng, C. T., Veale, D. J., Murphy, T. B., Rudd, P. M., & FitzGerald, O. (2013) Glycosylation status of serum in inflammatory arthritis in response to anti-TNF treatment. Rheumatology, 52, 1572–1582. DOI: 10.1093/rheumatology/ket189.
Coutinho, P. M., Deleury, E., Davies, G. J., & Henrissat, B. (2003) An evolving hierarchical family classification for glycosyltransferases. Journal of Molecular Biology, 328, 307–317. DOI: 10.1016/s0022-2836(03)00307-3.
Dwek, R. A., Butters, T. D., Platt, F. M., & Zitzmann, N. (2002) Targeting glycosylation as a therapeutic approach. Nature Reviews Drug Discovery, 1, 65–75. DOI: 10.1038/nrd708.
Herve du Penhoat, P. C. M., & Perlin, A. S. (1974) A carbon 13 N.M.R. spectral study of dpsicose: anomeric and ringform equilibria of solutions of 2-hexaneuloses. Carbohydrate Research, 36, 111–120. DOI: 10.1016/s0008-6215(00)81996-5.
Herzig, J., Nudelman, A., Gottlieb, H. E., & Fischer, B. (1986) Studies in sugar chemistry. 2. A simple method for O-deacetylation of polyacylated sugars. Journal of Organic Chemistry, 51, 727–730. DOI: 10.1021/jo00355a026.
Hirsch, J., Koóš, M., & Tvaroška, I. (2009) Synthesis of saccharide precursors for preparation of potential inhibitors of glycosyltranferases. Chemical Papers, 63, 329–335. DOI: 10.2478/s11696-009-0008-8.
Kelley, J. L., McLean, E. W., Crouch, R. C., Averett, D. R., & Tuttle, J. V. (1995) [[(Guaninylalkyl)phosphinico]methyl] phosphonic acids. Multisubstrate analog inhibitors of human erythrocyte purine nucleoside phosphorylase. Journal of Medicinal Chemistry, 38, 1005–1014. DOI: 10.1021/jm00006a020.
Lairson, L. L., Henrissat, B., Davies, G. J., & Withers, S. G. (2008) Glycosyltransferases: Structures, functions, and mechanisms. Annual Reviews of Biochemistry, 77, 521–555. DOI: 10.1146/annurev.biochem.76.061005.092322.
Marquardt, T., & Freeze, H. (2001) Congenital disorders of glycosylation: Glycosylation defects in Man and biological models for their study. Biological Chemistry, 382, 161–177.
Matsumura, F., Tatsumi, S., Oka, N., & Wada, T. (2010) Rapid glycosylations under extremely mild acidic conditions. Use of ammonium salts to activate glycosyl phosphites via P-protonation. Carbohydrate Research, 345, 1211–1215. DOI: 10.1016/j.carres.2010.03.009.
Matulic-Adamic, J., Haeberli, P., & Usman, N. (1995) Synthesis of 5′-deoxy-5′-difluoromethyl phosphonate nucleotide analogs. Journal of Organic Chemistry, 60, 2563–2569. DOI: 10.1021/jo00113a040.
Montreuil, J., Vliegenthart, J. F. G., & Schachter, H. (1996) Glycoproteins and disease. Amsterdam, The Netherlands: Elsevier.
Neuberger, A., & Deenen, L. L. M. (1995) Glycoproteins. (Vol. 29a). Amsterdam, The Netherlands: Elsevier.
Pauling, L. (1948) Chemical achievement, and hope for the future. American Scientist, 36, 51–58.
Perez, S., & Tvaroska, I. (2014) Carbohydrate-protein interactions — molecular modeling insights. Advances in Carbohydrate Chemistry and Biochemistry. submitted
Prisbe, E. J., Smejkal, J., Verheyden, J. P. H., & Moffatt, J. G. (1976) Halo sugar nucleosides. 5. Synthesis of angustmycin A and some base analogues. Journal of Organic Chemistry, 41, 1836–1846. DOI: 10.1021/jo00872a034.
Raab, M., Kozmon, S., & Tvaroška, I. (2005) Potential transition-state analogs for glycosyltransferases. Design and DFT calculations of conformational behavior. Carbohydrate Research, 340, 1051–1057. DOI: 10.1016/j.carres.2005.01.041.
Schramm, V. L. (1998) Enzymatic transition states and transition state analog design. Annual Reviews of Biochemistry, 67, 693–720. DOI: 10.1146/annurev.biochem.67.1.693.
Smith, M., Rammler, D. H., Goldberg, I. H., & Khorana, H. G. (1962) Studies on polynucleotides. XIV1 Specific synthesis of the C3″-C5″ interribonucleotide linkage. syntheses of uridylyl-(3″ → 5″)-uridine and uridylyl-(3″ → 5″)-adenosine2. Journal of the American Chemical Society, 84, 430–440. DOI: 10.1021/ja00862a023.
Stevens, F. J., & Argon, Y. (1999) Protein folding in the ER. Seminars in Cell & Developmental Biology, 10, 443–454. DOI: 10.1006/scdb.1999.0315.
Tvaroska, I. (2014) Atomistic insight into the catalytic mechanism of glycosyltransferases by combined quantum mechanics/molecular mechanics (QM/MM) methods. Carbohydrate Research. (in press).
Weis, W. I., Taylor, M. E., & Drickamer, K. (1998) The C-type lectin superfamily in the immune system. Immunologicals Reviews, 163, 19–34. DOI: 10.1111/j.1600-065X.1998.tb01185.x.
Wolfenden, R. (1969) Transition state analogues for enzyme catalysis. Nature, 223, 704–705. DOI: 10.1038/223704a0.
Yoshihara, A., Haraguchi, S., Gullapalli, P., Rao, D., Morimoto, K., Takata, G., Jones, N., Jenkinson, S. F., Wormald, M. R., Dwek, R. A., Fleet, G. W. J., & Izumori, K. (2008) Isomerization of deoxyhexaneoses: green bioproduction of 1-deoxy-dtagatose from lfucose and of 6-deoxy-dtagatose from dfucose using Enterobacter agglomerans strain 221e. Tetrahedron: Assymetry, 19, 739–745. DOI: 10.1016/j.tetasy.2008.02.013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baráth, M., Lin, CH., Tvaroška, I. et al. Development of transition state analogue inhibitors for N-acetylglycosyltransferases bearing dpsico- or dtagatofuranose scaffolds. Chem. Pap. 69, 348–357 (2015). https://doi.org/10.1515/chempap-2015-0063
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/chempap-2015-0063