Abstract
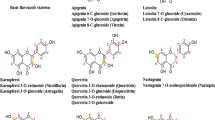
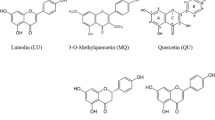
Protein glycation leads to the formation of advanced glycation end-products (AGEs), which contribute to the pathogenesis of diabetic complications. The structure-activity relationship of dietary flavonoids for inhibiting the glycation of bovine serum albumin (BSA) in vitro was subjected to a detailed investigation. The structure-activity relationship revealed that: 1) the hydroxylation on ring B of the flavones enhanced the inhibition and the hydroxyl groups at the C-5 and C-7 positions of flavones favoured the inhibition; 2) the optimal number of hydroxyl groups on ring B of the flavonols was one (at the C-3 position) and the methylation of flavonols weakened the inhibition; 3) the methoxylation at the C-6 position and methylation at C-4′ position of genistein clearly enhanced the inhibition; 4) the hydroxyl groups at the C-5 and C-7 positions of flavanones were in favour of the inhibition; 5) the glycosylation of flavonoids significantly weakened the inhibition. Obvious linear affinity-activity relationships exist between the BSA-flavonoid interaction and flavonoids as BSA glycation inhibitors (R2 = 0.76585). The flavonoids with a higher affinity to BSA exhibited a stronger inhibition of the glycation of BSA.
Similar content being viewed by others
References
Andrae-Marobela, K., Ghislain, F. W., Okatch, H., & Majinda, R. R. T. (2013) Polyphenols: A diverse class of multi-target anti-HIV-1 agents. Current Drug Metabolism, 14, 392–413. DOI: 10.2174/13892002113149990095.
Barnaby, O. S., Cerny, R. L., Clarke, W., & Hage, D. S. (2011) Comparison of modification sites formed on human serum albumin at various stages of glycation. Clinica Chimica Acta, 412, 277–285. DOI: 10.1016/j.cca.2010.10.018.
Cao, H., Shi, Y. J., & Chen, X. Q. (2013) Advances on the interaction between tea catechins and plasma proteins: Structure-affinity relationship, influence on antioxidant activity, and molecular docking aspects. Current Drug Metabolism, 14, 446–450. DOI: 10.2174/1389200211314040007.
Deng, G. F., Xu, X. R., Zhang, Y., Li, D., Gan, R. Y., & Li, H. B. (2013) Phenolic compounds and bioactivities of pigmented rice. Critical Reviews in Food Science and Nutrition, 53, 296–306. DOI: 10.1080/10408398.2010.529624.
Hughes, L.A. E., Arts, I.C. W., Ambergen, T., Brants, H. A. M., Dagnelie, P. C., Goldbohm, R. A., van den Brandt, P. A., & Weijenberg, M. P. (2008) Higher dietary flavone, flavonol, and catechin intakes are associated with less of an increase in BMI over time in women: a longitudinal analysis from the Netherlands Cohort Study The American Journal of Clinical Nutrition, 88, 1341–1352. DOI: 10.3945/ajcn.2008.26058.
Jang, D. S., Lee, Y. M., Jeong, I. H., & Kim, J. S. (2010) Constituents of the flowers of Platycodon grandiflorum with inhibitory activity on advanced glycation end products and rat lens aldose reductase in vitro. Archives of Pharmacal Research, 33, 875–880. DOI: 10.1007/s12272-010-0610-x.
Johnson, M. H., Gonzalez de Mejia, E., Fan, J. F., Lila, M. A., & Yousef, G. G. (2013) Anthocyanins and proanthocyanidins from blueberry-blackberry fermented beverages inhibit markers of inflammation in macrophages and carbohydrate-utilizing enzymes in vitro. Molecular Nutrition & Food Research, 57, 1182–1197. DOI: 10.1002/mnfr.201200678.
Jung, S.H., Lee, J.M., Lee, H.J., Kim, C.Y., Lee, E.H., & Um, B. H. (2007) Aldose reductase and advanced glycation endproducts inhibitory effect of Phyllostachys nigra. Biological and Pharmaceutical Bulletin, 30, 1569–1572. DOI: 10.1248/bpb.30.1569.
Liu, Y. J., Zhan, J., Liu, X. L., Wang, Y., Ji, J., & He, Q. Q. (2014) Dietary flavonoids intake and risk of type 2 diabetes: A meta-analysis of prospective cohort studies. Clinical Nutrition, 33, 59–63. DOI: 10.1016/j.clnu.2013.03.011.
Matsuda, H., Wang, T., Managi, H., & Yoshikawa, M. (2003) Structural requirements of flavonoids for inhibition of protein glycation and radical scavenging activities. Bioorganic & Medicinal Chemistry, 11, 5317–5323. DOI: 10.1016/j.bmc.2003.09.045.
Rondeau, P., & Bourdon, E. (2011) The glycation of albumin: Structural and functional impacts. Biochimie, 93, 645–658. DOI: 10.1016/j.biochi.2010.12.003.
Thornalley, P. J., Langborg, A., & Minhas, H. S. (1999) Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochemical Journal, 344, 109–116.
van Dam, R. M., Naidoo, N., & Landberg, R. (2013) Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases: review of recent findings. Current Opinion on Lipidology, 24, 25–33. DOI: 10.1097/MOL.0b013e32835bcdff.
Wang, Y. L., Zhao, Y., Yang, F., Yuan, Y. M., Wang, H., & Xiao, J. B. (2012) Influences of glucose on the dietary hydroxyflavonoids-plasma proteins interaction. Journal of Agricultural & Food Chemistry, 60, 12116–12221. DOI: 10.1021/jf303094e.
Wedick, N. M., Pan, A., Cassidy, A., Rimm, E. B., Sampson, L., Rosner, B., Willett, W., Hu, F. B., Sun, Q., & van Dam, R. M. (2012) Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. The American Journal of Clinical Nutrition, 95, 925–933. DOI: 10.3945/ajcn.111.028894.
Wild, S., Roglic, G., Green, A., Sicree, R., & King, H. (2004) Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care, 27, 1047–1053. DOI: 10.2337/diacare.27.5.1047.
World Health Organization (2013) Diabetes. Fact sheet no. 312. Retrieved March 2013, from http://www.who.int/mediacentre/factsheets/fs312/en/index.html
Xiao, J. B., & Kai, G. Y. (2012) A review of dietary polyphenolplasma protein interactions: Characterization, influence on the bioactivity, and structure-affinity relationship. Critical Reviews in Food Science and Nutrition, 52, 85–101. DOI: 10.1080/10408398.2010.499017.
Xiao, J. B. (2013) Polyphenol-plasma proteins interaction: Its nature, analytical techniques, and influence on bioactivities of polyphenols. Current Drug Metabolism, 14, 367–368. DOI: 10.2174/1389200211314040001.
Xiao, J. B., Ni, X. L., Kai, G. Y., & Chen, X. Q. (2013a) A review on structure-activity relationship of dietary polyphenols inhibiting a-amylase. Critical Reviews in Food Science and Nutrition, 53, 497–506. DOI: 10.1080/10408398.2010.548108.
Xiao, J. B., Kai, G. Y., Yamamoto, K., & Chen, X. Q. (2013b) Advance in dietary polyphenols as α-glucosidases inhibitors: A review on structure-activity relationship aspect. Critical Reviews in Food Science and Nutrition, 53, 818–836. DOI: 10.1080/10408398.2011.561379.
Xiao, J. B., & Högger, P. (2014) Dietary polyphenols and type 2 diabetes: current insights and future perspectives. Current Medicinal Chemistry, 21, 1–16. DOI: 10.2174/0929867321666140706130807.
Xiao, J. B., Chen, T. T., & Cao, H. (2014) Flavonoid glycosylation and biological benefits. Biotechnology Advances, in press. DOI: 10.1016/j.biotechadv.2014.05.004.
Xiao, J. B., Ni, X. L., Kai, G. Y., & Chen, X. Q. (2015) Advance in dietary polyphenols as aldose reductases inhibitors: Structure-activity relationship aspect. Critical Reviews in Food Science and Nutrition, 55, 16–31. DOI: 10.1080/10408398.2011.584252.
Xie, Y. X., & Chen, X. Q. (2013) Structures required of polyphenols for inhibiting advanced glycation end products formation. Current Drug Metabolism, 14, 414–431. DOI: 10.2174/1389200211314040005.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Q., Chen, TT. & Cao, H. Flavonoids inhibiting glycation of bovine serum albumin: affinity-activity relationship. Chem. Pap. 69, 409–415 (2015). https://doi.org/10.1515/chempap-2015-0050
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/chempap-2015-0050