Abstract
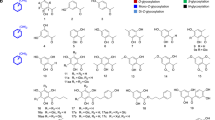
Based on rational design of the transition state analog inhibitors of glycosyltransferases, four model glycomimetics of this type, viz. benzyl 2-thio-α-dfructofuranoside 1-diethylphosphate (XIa), its β-anomer (XIb), and their ethyl 2-thio analogs — α-anomer (XIIa) and β-anomer (XIIb), were synthesized. In addition, fourteen precursors arising during the synthesis of the desired final model compounds (XI and XII), partially or fully acetylated benzyl and/or ethyl 2-thiofructofuranoside 1-diethyl phosphates, were isolated and characterized with the aim to prepare complete series of glycomimetics, representing donor UDP-GlcNAc designated for biological assays on human GnT’s, viz. GnT-I, Core2GnT, and GnT-V.
Similar content being viewed by others
References
Beyer, T. A., Sadler, J. E., Rearick, J. I., Paulson, J. C., & Hill, R. L. (1981) Glycosyltransferases and their use in assessing oligosaccharide structure and structure-function relationship. Advances in Enzymology and Related Areas of Molecular Biology, 52, 23–175. DOI: 10.1002/9780470122976.ch2.
Breton, C., Fournel-Gigleux, S., & Palcic, M. M. (2012) Recent structures, evolution and mechanisms of glycosyltransferases. Current Opinion in Structural Biology, 22, 540–549. DOI: 10.1016/j.sbi.2012.06.007.
Hadley, B., Maggioni, A., Ashikov, A., Day, C. J., Haselhorst, T., & Tiralongo, J. (2014) Structure and function of nucleotide sugar transporters: Current progress. Computational and Structural Biotechnology Journal, in press. DOI: 10.1016/j.csbj.2014.05.003.
Herzig, J., Nudelman, A., Gottlieb, H. E., & Fischer, B. (1986) Studies in sugar chemistry. 2. A simple method for O-deacetylation of polyacylated sugars. Journal of Organic Chemistry, 51, 727–730. DOI: 10.1021/jo00355a026.
Hirsch, J., Koóš, M., & Tvaroška, I. (2009) Synthesis of saccharide precursors for preparation of potential inhibitors of glycosyltransferases. Chemical Papers, 63, 329–335. DOI: 10.2478/s11696-009-0008-8.
Kleene, R., & Berger, E. G. (1993) The molecular and cell biology of glycosyltransferases. Biochimica et Biophysica Acta (BBA) — Reviews on Biomembranes, 1154, 283–325. DOI: 10.1016/0304-4157(93)90003-7.
Marquardt, T., & Freeze, H. (2001) Congenital disorders of glycosylation: Glycosylation defects in man and biological models for their study. Biological Chemistry, 382, 161–177.
Montreuil, J., Vliegenthart, J. F. G., & Schachter, H. (1995) Glycoproteins (Series: New comprehensive biochemistry, Vol. 29, Part A). Amsterdam, The Netherlands: Elsevier.
Montreuil, J., Vliegenthart, J. F. G., & Schachter, H. (1996) Glycoproteins and disease (Series: New comprehensive biochemistry, Vol. 30). Amsterdam, The Netherlands: Elsevier.
Raab, M., Kozmon, S., & Tvaroška, I. (2005) Potential transition-state analogs for glycosyltransferases. Design and DFT calculations of conformational behavior. Carbohydrate Research, 340, 1051–1057. DOI: 10.1016/j.carres.2005.01.041.
Sears, P., & Wong, C. H. (1999) Carbohydrate mimetics: A new strategy for tackling the problem of carbohydrate-mediated biological recognition. Angewandte Chemie International Edition, 38, 2300–2324. DOI: 10.1002/(SICI)1521-3773(19990816)38:16<2300::AID-ANIE2300>3.0.CO;2-6.
Waldscheck, B., Strieff, M., Notz, W., Kinzy, W., & Schmidt, R. R. (2001) α(1-3)-Galactosyltransferase inhibition based on a new type of disubstrate analogue. Angewandte Chemie International Edition, 40, 4007–4011. DOI: 10.1002/1521-3773(20011105)40:21<4007::AID-ANIE4007>3.0.CO;2-F.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baráth, M., Koóš, M., Tvaroška, I. et al. Synthesis of potential inhibitors of glycosyltransferases representing UDP-GlcNAc. Chem. Pap. 69, 339–347 (2015). https://doi.org/10.1515/chempap-2015-0017
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/chempap-2015-0017