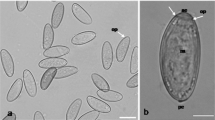
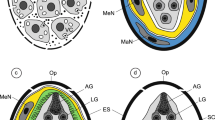
Abstract
Despite their tremendous diversity and their medical and veterinary importance, details of egg ultrastructure among the digenean trematodes has been studied rather little. The available literature is spread over several decades and several species, but has not been adequately reviewed to reveal patterns of similarity and divergence. We present this review to synthesize and analyse what is known from the available literature reporting studies using both transmission electron microscopy (TEM) and scanning electron microscopy (SEM). To support our general review of existing literature, we also have synthesized our own previously published descriptions, and present herein our new previously unpublished data. From these new electron micrographs, we provide a comparative analysis of the intrauterine eggs of four digenean species, representing four genera and three families of the superfamily Microphalloidea, collected from four different host wildlife species in four European countries: 1) Mediogonimus jourdanei (Prosthogonimidae) from Myodes glareolus (Mammalia: Rodentia), collected in France; 2) Maritrema feliui (Microphallidae) from Crocidura russula (Mammalia: Soricimorpha), collected in Spain; 3) Brandesia turgida (Pleurogenidae) from Pelophylax ridibundus (Amphibia: Anura: Ranidae), collected in Russia; and 4) Prosotocus confusus (Pleurogenidae) from Rana lessonae (Amphibia: Anura: Ranidae), collected in Belarus. All were studied by preparing whole worms by various techniques for TEM, so that eggs could be studied in situ within the uterus of the parent worm. Based on the literature review and the new data presented here, we describe basic similarities in patterns of embryogenesis and egg formation among all trematode species, but substantial variations in timing of larvigenesis, sculpturing of egg shell surfaces, and some other features, especially including accessory cocoon coverings outside the egg shells of B. turgida and P. confusus. In the future, many more studies are needed to explore egg ultrastructure in other digenean taxa, to explore potential phylogenetic patterns in egg development and structure, and to correlate structure with function in the life cycle.
Similar content being viewed by others

References
Ashton P.D., Harrop R., Shah B., Wilson R.A. 2001. The schistosome egg: development and secretions. Parasitology, 122, 329–338. DOI: 10.1017/S0031182001007351
Björkman N., Thorsell W. 1963. On the fine morphology of the formation of egg-shell globules in the vitelline glands of the liver fluke (Fasciola hepatica L.). Experimental Cell Research, 32, 153–156
Born-Torrijos A., Holzer A.S., Raga J.A., van Beest G.S., Yoneva A. 2017. Description of embryonic development and ultrastructure in miracidia of Cardiocephaloides longicollis (Digenea, Strigeidae) in relation to active host finding strategy in a marine environment. Journal of Morphology, 278, 1137–1148. DOI: 10.1002/jmor.20700
Bundy D.A.P. 1981. The surface ultrastructure of the egg capsule of Transversotrema patialense (Transversotrematidae: Digenea). International Journal for Parasitology, 11, 19–22. DOI: 10.1016/0020-7519(81)90021-7
Burt M.D.B. 1986. Early morphogenesis in the Platyhelminthes with special reference to egg development and development of ces-tode larvae. International Journal for Parasitology, 17, 241–253. DOI: 10.1016/0020-7519(87)90047-6
Cable J., Tinsley R.C. 1991. Intrauterine larval development of the polystomatid monogeneans, Pseudodiplorchis americanus and Neodiplorchis scaphiopodis. Parasitology, 103, 253–266. DOI:10.1017/S0031182000059539
Cable J., Harris P.D., Tinsley R.C. 1996. Ultrastructural adaptations for viviparity in the female reproductive system of gyrodactylid monogeneans. Tissue and Cell, 28, 515–526
Cable J., Tocque K., Tinsley R.C. 1997. Histological analysis of the egg capsule of the ovoviviparous polystomatid monogenean, Pseudodiplorchis americanus. International Journal for Parasitology, 27, 1075–1080. DOI: 10.1016/S0020-7519(97)00068-4
Colhoun L.M., Fairweather I. Brennan G.P. 1998. Observations on the mechanism of eggshell formation in the liver fluke, Fasciola hepatica. Parasitology, 116, 555–567.
Conn D.B. 1985. Fine structure of the embryonic envelopes of Oochoristica anolis (Cestoda: Linstowiidae). Zeitschrift für Parasitenkunde, 71, 639–648. DOI: 10.1007/BF00925597
Conn D.B. 1988. Are vitelline cell globules a mechanism for polyspermy prevention in cyclophyllidean cestodes? Transactions of the American Microscopical Society, 107, 106
Conn D.B. 1999. Ultrastructure of the embryonic envelopes and associated maternal structures of Distoichometra bufonis (Platyhelminthes: Cestoidea: Nematotaeniidae). Acta Parasitologica, 43, 4–10
Conn D.B. 2000. Atlas of Invertebrate Reproduction and Development. 2nd ed. New York, John Wiley & Sons, Inc., 3115 pp. ISBN: 0-471-23796-5
Conn D.B. 2007a. Life cycles and biogeography of fish parasites: recent advances and future directions. Parassitologia, 49(Suppl. 2), 282
Conn D.B. 2007b. Recent advances in the reproductive and developmental biology of Trematoda and Cestoda. Wiadomości Parazytologiczne, 53(Suppl), 6
Conn D.B. 2010. Invasion strategies of trematodes involving activity of the life-cycle stages between cercaria and metacercaria. In: I. Hodová and B. Koubková (eds.). 18th Helminthological Days 2010: Book of Abstracts. Brno, Czech Republic, MUNI Press, Masaryk University, 22–23. ISBN: 978-80-210-5244-4
Conn D.B. 2016. Redefining parasite life cycles through epidemiology, pathology and developmental biology. Annals of Parasitology, 62(Suppl.), 7
Conn D.B., Conn D.A. 1995. Experimental infestation of zebra mussels, Dreissena polymorpha (Mollusca: Bivalvia), by metacercariae of Echinoparyphium sp. (Platyhelminthes: Trematoda). Journal of Parasitology, 81, 304–305
Conn D.B., Etges F.J. 1983. Inhibition of egg production in an anomalous Plagitura salamandra Holl, 1928 (Trematoda: Plagiorchiidae). Journal of Parasitology, 69, 784–786
Conn D.B., Etges F.J. 1984. Fine structure and histochemistry of the parenchyma and uterine egg capsules of Oochoristica anolis (Cestoda: Linstowiidae). Zeitschrift für Parasitenkude, 70, 769–779. DOI: 10.1007/BF00927130
Conn D.B., Etges F.J., Sidner R.A. 1984. Fine structure of the gravid paruterine organ and embryonic envelopes of Mesocestoides lineatus (Cestoda). Journal of Parasitology, 70, 68–77
Conn D.B., Goater C.P., Bray D. 2008. Developmental and functional ultrastructure of Ornithodiplostomum ptychocheilus diplostomula (Trematoda: Strigeoidea) during invasion of the brain of the fish intermediate host, Pimephales promelas. Journal of Parasitology, 94, 635–642. DOI: 10.1645/GE-1421.1
Conn D.B., Świderski Z. 2008. A standardised terminology of the embryonic envelopes and associated developmental stages of tapeworms (Platyhelminthes: Cestoda). Folia Parasitologica, 55, 42–52
Conn D.B., Świderski Z., Młocicki D. 2007. A review and synthesis of ultrastructure and development of embryonic structures in parasitic neodermatan flatworms. Abstracts of the 11th International Congress on Invertebrate Reproduction and Development, Panama City, Panama, p. 53
Ditrich O., Giboda M., Scholz T., Beer S.A. 1992. Comparative morphology of eggs of the Haplorchiinae (Trematoda: Heterophyidae) and some other medically important heterophyid and opisthorchiid flukes. Folia Parasitologica (Praha), 39, 123–132.
Eklu-Natey D.T., Świderski Z., Moczon T., Striebel H.P., Huggel H. 1982. Ultrastructure and histochemistry of egg-shell formation in Schistosoma haematobium. Abstracts of the 5th International Congress of Parasitology, 7–14 August, 1982, Toronto, Canada. Molecular and Biochemical Parasitology, Suppl. 5, 708
Ford J.W., Blankespoor D. 1979. Scanning electron microscopy of the eggs of three human schistosomes. International Journal for Parasitology, 9, 141–145. DOI: 10.1016/0020-7519(79)90105-X
Fujino T., Higo H., Ishii Y., Saito S., Chen E.R. 1989. Comparative studies on two similar species of Haplorchis and Metagonimus (Trematoda: Heterophyidae)—Surface ultrastructure of adults and eggs. Proceedings of the Helminthological Society of Washington, 56, 35–41
Fujino T., Nakano T., Washioka H., Tonosaki A., Ichikawa H., Fried B. 2000. Comparative ultrastructure of eggs in Echinostoma paraensei, E. caproni, and E. trivolvis (Trematoda: Echinostomatidae). Parasitology Research, 86, 427–430. DOI: 10.1007/s004360050689
Goater C.P., Bray D., Conn D.B. 2005. Cellular aspects of early development of Ornithodiplostomum ptychocheilus metacer-cariae in the brain of fathead minnows, Pimephales promelas. Journal of Parasitology, 91, 814–821. DOI: 10.1645/GE-3485.1
Holy J.M., Wittrock D.D. 1986. Ultrastructure of the female reproductive organs (ovary, vitellaria, and Mehlis’ gland) of Halipegus eccentricus (Trematoda: Derogenidae). Canadian Journal of Zoology, 1986, 64, 2203–2212. DOI: 10.1139/z86-334
Irwin S.W.B., Threadgold L.T. 1970. Electron-microscope studies on Fasciola hepática VIII. The development of the vitelline cells. Experimental Parasitology, 28, 399–411. DOI: 10.1016/0014-4894(70)90108-6
Irwin S.W.B., Threadgold L.T. 1972 Electron microscope studies of Fasciola hepatica. X. Egg formation. Experimental Parasitology, 31, 321–331. DOI: 10.1016/0014-4894(72)90093-8
Jones M.K., Bong S.H., Green K.M., Holmes P., Duke M., Lukas A., McManus D.P. 2008. Correlative and dynamic imaging of the hatching biology of Schistosoma japonicum from eggs prepared by high pressure freezing. PLoS Neglected Tropical Diseases, 2, e334. DOI: 10.1371/journal.pntd. 0000334
Jurberg A.D., Gonçalves T., Costa T.A., de Mattos A.C.A., Pascarelli B.M., de Manso P.P.A., et al. 2009. The embryonic development of Schistosoma mansoni eggs: proposal for a new staging system. Development Genes and Evolution, 219, 219–234. DOI: 10.1007/s00427-009-0285-9
Justine J.-L., Mattei X. 1984. Ultrastructural observations on the spermatozoon, oocyte and fertilization process in Gonapodasmius, a gonochoristic trematode (Trematoda: Digenea: Didymozoidae). Acta Zoologica (Stockholm), 65, 171–177. DOI: 10.1111/j.1463-6395.1984.tb00822.x
Karatayev A.Y., Mastitsky S.E., Burlakova L.E., Karatayev V.A., Hajduk M.M., Conn D.B. 2012. Exotic molluscs in the Great Lakes host epizootically important trematodes. Journal of Shellfish Research, 31, 885–894
Khampoosa P., Jones M.K., Lovas E.M., Srisawangwong T., Laha T., Piratae S., et al. 2012. Light and electron microscopy observations of embryogenesis and egg development in the human liver fluke, Opisthorchis viverrini (Platyhelminthes, Digenea). Parasitology Research, 110, 799–808. DOI: 10. 1007/s00436-011-2557-3
Korneva Zh.V. 2001. Vitellogenesis and capsule formation during embryogenesis in Triaenophorus nodulosus (Cestoda, Pseudophyllidea, Triaenophoridae). Zoologicheskii Zhurnal, 80, 1422–1428
Krejci K.G., Fried B. 1994. Light and scanning electron microscopic observations of the eggs, daughter rediae, cercariae, and encysted metacercariae of Echinostoma trivolvis and E. caproni. Parasitology Research, 80, 42–47. DOI: 10.1007/BF00932622
Krupa P.L. 1974. Ultrastructural topography of a trematode egg shell. Experimental Parasitology, 35, 244–247. DOI: 10.1016/0014-4894(74)90028-9
Levron C., Scholz T., Vancová M., Kuchta R., Conn D.B. 2016. Ultrastructure of embryonated eggs of the cestode Gyrocotyle urna (Gyrocotylidea) using cryomethods. Zoomorphology, 135, 279–289. DOI: 10.1007/s00435-016-0310-2
Martin T.R., Conn D.B. 1990. The pathogenicity, localization and cyst structure of echinostomatid metacercariae (Trematoda) infecting the kidneys of the frogs Rana clamitans and Rana pipiens. Journal of Parasitology, 76, 414–419
Meepool A., Wanichanon C., Viyanant V., Sobhon P. 2006. Development and roles of vitelline cells in egg shell formation in Fasciola gigantica. Invertebrate Reproduction & Development, 49, 1–2, 9–17. DOI: 10.1080/07924259.2006.9652189
Młocicki D., Świderski Z., Bruňanská M., Conn D.B. 2010a. Functional ultrastructure of the hexacanth larvae in the bothrioce-phalidean cestode Eubothrium salvelini (Schrank, 1790) and its phylogenetic implications. Parasitology International, 59, 539–548. DOI: 10.1016/j.parint.2010.07.001
Młocicki D., Świderski Z., Mackiewicz J.S., Ibraheem M.H. 2010b. Ultrastructure of intrauterine eggs: evidence of early ovovi-viparity in the caryophyllidean cestode Wenyonia virilis Woodland, 1923. Acta Parasitologica, 55, 349–358. DOI: 10.2478/s11686-010-0044-0
Neill P.J.G., Smith J.H., Doughty B.L., Kemp M. 1988. The ultrastructure of the Schistosoma mansoni egg. American Journal of Tropical Medicine and Hygiene, 39, 52–65. DOI: 10.4269/ajtmh.1988.39.52
Orido Y. 1988. Fertilization and oogenesis of the lung fluke, Paragonimus ohirai (Trematoda: Troglotrematidae). International Journal for Parasitology, 18, 973–980. DOI: 10.1016/0020-7519(88)90180-4
Pinheiro J., Franco-Acuña D.O., Oliveira-Menezes A., Brandolini S.V.P.B., Adnet F.A.O., Lopes Torres E.J., et al. 2015. Additional study of the morphology of eggs and miracidia of Eurytrema coelomaticum (Trematoda). Helminthologia, 52, 244–251. DOI: 10.1515/helmin-2015-0039
Poddubnaya L.G., Mackiewicz J.S., Bruňanská M., Scholz T. 2005. Fine structure of the female reproductive ducts of Cyathocephalus truncatus (Cestoda: Spathebothriidea), from salmonid fish. Folia Parasitologica, 52, 323–338
Sato M., Oh M., Sakoda K., Nakashio H., Noguchi T. 1966. Electron microscopy of the egg-shell formation in the lung fluke, Paragonimus miyazakii. Journal of Electron Microscopy, 15, 286–287
Scholz T., Ditrich O., Giboda M. 1992 Study on the surface morphology of the developmental stages of the liver fluke, Opisthorchis viverrini (Trematoda: Opisthorchiidae). Annales de Parasitologie Humaine et Comparée, 67, 82–90. DOI: 10.1051/parasite/199267382
Shin D.H., Lim D.-S., Choi K.-J., Oh C.S., Kim M.J., Lee I.S., et al. 2009. Scanning electron microscope study of ancient parasite eggs recovered from Korean mummies of the Joseon Dynasty. Journal of Parasitology, 95, 137–145. DOI: 10.1645/GE-1588.1
Shinn G.L. 1993. Formation of egg capsules by flatworms (phylum Platyhelminthes). Transactions of the American Microscopical Society, 112, 18–34
Świderski Z. 1984. Embryonic development of Schistosoma mansoni. South African Journal of Science, 80, 434
Świderski Z. 1985. Embryonic development of Schistosoma mansoni and S. haematobium: egg envelope formation. South African Journal of Science, 81, 43–44
Świderski Z. 1986. Schistosoma mattheei: egg-shell degeneration in the liver of Praomys (Mastomys) natalensis during chronic infection. In: (Eds. T. Imura, S. Maruse and T. Suzuki) Proceedings of the 11th International Congress on Electron Microscopy, August 31–September 7, 1986, Kyoto, Japan, 3567–3568
Świderski Z. 1988. Ultrastructure of schistosome eggs. Proceedings of the 4th Asia Pacific Conference and Workshop on Electron Microscopy, July 26–August 4, 1988, Bangkok, Thailand, 555–556
Świderski Z. 1993. Functional ultrastructure and origin of egg envelopes surrounding coracidia of Bothriocephalus clavibothrium (Cestoda, Pseudophyllidea). Proceedings, International 5th Beijing Conference and Exhibition on Instrumental Analysis, October 9–12, 1993, Beijing, China, 125–126
Świderski Z. 1994a. Origin, differentiation and ultrastructure of egg envelopes surrounding the miracidia of Schistosoma mansoni. Acta Parasitologica, 39, 64–72
Świderski Z. 1994b. Origin, differentiation and ultrastructure of egg envelopes surrounding the coracidia of Bothriocephalus clavibothrium (Cestoda, Pseudophyllidea). Acta Parasitologica, 39, 73–81
Świderski Z. 1994c. Homology and analogy in egg envelopes surrounding miracidia of Schistosoma mansoni and coracidia of Bothriocephalus clavibothrium. Acta Parasitologica, 39, 123–130
Świderski Z. 1996. Comparative studies on the ultrastructure, homology and analogy of egg envelopes in trematodes and cestodes. Wiadomości Parazytologiczne, 42, 81–96
Świderski Z., Conn D.B. 2000. Egg formation in trematodes and cestodes: comparative ultrastructural studies. Acta Parasitologica, 45, 158
Świderski Z., Conn D.B. 2001. Comparative ultrastructural studies on egg formation in some species of trematodes and cestodes. In Proceedings of the 9th International Congress on Invertebrate Reproduction and Development, Grahamstown, South Africa. pg. 93
Świderski Z., Conn D.B. 2004. The differentiation and functional ultrastructure of the parenchymatic egg envelopes: Their role in cestode embryogenesis. Wiadomosci Parazytologiczne, 50 (Suppl), 116–117
Świderski Z., Conn D.B. 2014. Comparative ultrastructure of the intrauterine eggs of four European trematodes. In: M. Oros and Z. Vasilková (eds.). V4 Parasitological Meeting: Parasites in the Heart of Europe. Slovak Society for Parasitology at SAS, Kosice, Slovakia. 27–28. ISBN: 978-80-968473-7-2
Świderski Z., Mackiewicz J.S. 2007. Ultrastructure of polylecithal and oligolecithal eggs of cestode parasites of fishes: comparative TEM study. Parassitologia, 49, 394
Świderski Z., Moser P., Eklu-Natey D.T. 1980. The fine structure of protective envelopes of the egg of Schistosoma mansoni. In: (Eds. P. Brederoo and W. De Priester) Electron Microscopy. Vol. 2. Leiden, The Netherlands, 218–219
Świderski Z., Bakhoum A.J.S., Młocicki D., Miquel J. 2010. Ultrastructural studies on egg envelopes surrounding the miracidia of Mediogonimus jourdanei Mas-Coma et Rocamora 1978 (Digenea, Microphalloidea, Prosthogonimidae). Acta Parasitologica, 55, 245–253. DOI: 10.2478/s11686-010-0031-5
Świderski Z., Poddubnaya L.G., Gibson D.I., Levron C., Młocicki D. 2011. Egg formation and the early embryonic development of Aspidogaster limacoides Diesing, 1835 (Aspidogastrea: Aspidogastridae), with comments on their phylogenetic significance. Parasitology International, 60, 371–380. DOI: 10.1016/j.parint.2011.06.006
Świderski Z., Poddubnaya L.G., Gibson D.I., Młocicki D. 2012. Advanced stages of embryonic development and cotylocidial morphogenesis in the intrauterine eggs of Aspidogaster lima-coides Diesing, 1835 (Aspidogastrea), with comments on their phylogenetic implications. Acta Parasitologica, 57, 131–148. DOI: 10.2478/s11686-012-0025-6
Świderski Z., Miquel J., Montoliu I., Feliu C., Gibson D.I. 2013a. Ultrastructure of the intrauterine eggs of the microphallid trematode Maritrema feliui: evidence of early embryonic development. Parasitology Research, 112, 3325–3333. DOI: 10.1007/s00436-013-3512-2
Świderski Z, Poddubnaya L.G., Zhokhov A.E., Miquel J., Gibson D.I. 2013b. An ultrastructural study of the egg wall surrounding the miracidium of the digenean Brandesia turgida (Brandes, 1888) (Plagiorchiida: Pleurogenidae), with the description of a unique cocoon-like envelope. Zoologischer Anzeiger, 253, 114–118. DOI: 10.1016/j.jcz.2013.09.001
Świderski Z., Poddubnaya L.G., Zhokhov A.E., Miquel J., Conn D.B. 2014. Ultrastructural evidence for completion of the entire miracidial maturation in intrauterine eggs of the digenean Brandesia turgida (Brandes, 1988) (Plagiorchiida: Pleurogenidae). Parasitology Research, 113, 1103–1111. DOI: 10.1007/s00436-013-3747-y
Świderski Z., Miquel J., Torres J., Conn D.B. 2015a. Ultrastructural study of the egg wall surrounding the developing miracidia of the digenean Prosotocus confusus (Looss, 1894) (Plagiorchiida: Pleurogenidae), with the description of a unique cocoon-like envelope. Parasitology Research, 114, 185–191. DOI: 10.1007/s00436-014-4177-1
Świderski Z., Miquel J., Feliu C., Conn D.B. 2015b. Functional ultrastructure of the parenchymatic capsules of the cestode Thysanotaenia congolensis (Cyclophyllidea, Anoplocephalidae, Inermicapsiferinae). Parasitology Research, 114, 297–303. DOI: 10.1007/s00436-014-4194-0
Świderski Z., Adalid R., Torres J., Miquel J. 2017a. Ultrastructure of egg envelopes surrounding the miracidia of Ityogonimus lorum (Dujardin, 1845) (Digenea, Brachylaimidae, Ityogoniminae). XX Congreso de la Sociedad Española de Parasitología, Tenerife, España. pg. 241
Świderski Z., Adalid R., Feliu C., Miquel J. 2017b. Ultrastructural evidence for completion of miracidial maturation in the intrauterine eggs of Ityogonimus lorum (Digenea, Brachylaimidae, Ityogoniminae). XX Congreso de la Sociedad Española de Parasitología, Tenerife, España. pg. 242.
Tesana S., Srisawangwonk T., Kaewkes S., Sithithaworn P., Kanla P., Arunyanart C. 1991. Egg shell morphology of the small eggs of human trematodes in Thailand. Southeast Asian Journal of Tropical Medicine and Public Health, 22, 631–636.
Tinsley R.C. 1983. Ovoviviparity in platyhelminth life-cycles. Parasitology, 86, 161–196. DOI: 10.1017/S0031182000050885
Wittrock D.D. 1982. Structure and origin of the egg shell of Quinqueserialis quinqueserialis (Trematoda: Notocotylidae). Zeitschrift für Parasitenkunde, 67, 37–44. DOI: 10.1007/BF00929512
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Conn, D.B., Świderski, Z. & Miquel, J. Ultrastructure of digenean trematode eggs (Platyhelminthes: Neoophora): A review emphasizing new comparative data on four European Microphalloidea. Acta Parasit. 63, 1–14 (2018). https://doi.org/10.1515/ap-2018-0001
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/ap-2018-0001