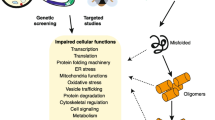
Abstract
Genetic experiments in mice, which are indispensable for studying the molecular basis of neurological disorders, have certain limitations that include slow pace and high costs. It is therefore not surprising that in recent years numerous neurological diseases have been modeled in genetically tractable organisms, including Drosophila, Caenorhabditis elegans, and yeast. Yeast models in particular have a special advantage with respect to genome-wide experimental approaches as a result of the completed sequencing of the genome, the availability of a collection of precise deletion mutants of every gene in the genome, and the rapidly evolving databases of yeast protein-protein interactions and gene expression patterns. These large and easily accessible bodies of information, coupled with the ease with which yeast can be manipulated genetically, have led to dissection of novel mechanisms of neurodegenerative disorders. In this review, we discuss how studies in yeast models have already resulted in significant insights into the understanding of neurodegenerative disorders that include prion disease, Parkinson’s disease, polyglutamine expansion disorders, Friedreich’s ataxia, and Batten disease.
Similar content being viewed by others
References
Anton L. C., Schubert U., Bacik I., et al. (1999) Intracellular localization of proteasomal degradation of a viral antigen. J. Cell Biol. 146, 113–124.
Babcock M., de Silva D., Oaks R., et al. (1997) Regulation of mitochondrial iron accumulation by Yfh1p, a putative homolog of frataxin. Science 276, 1709–1712.
Bailleul P. A., Newnam G. P., Steenbergen J. N., and Chernoff, Y. O. (1999) Genetic study of interactions between the cytoskeletal assembly protein sla1 and prion-forming domain of the release factor Sup35 (eRF3) in Saccharomyces cerevisiae. Genetics. 153, 81–94.
Bates G. P., Mangiarini L, and Davies, S. W. (1998) Transgenic mice in the study of polyglutamine repeat expansion diseases (review). Brain Pathol. 8, 699–714.
Bence N. F., Sampat R. M., and Kopito, R. R. (2001) Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292, 1552–1555.
Beranger F., Mange A., Goud B., and Lehmann, S. (2002) Stimulation of PrP(C) retrograde transport toward the endoplasmic reticulum increases accumulation of PrP(Sc) in prion-infected cells. J. Biol. Chem. 277, 38972–38977.
Bhattacharyya S, Rolfsmeier M. L., Dixon M. J., Wagoner K, and Lahue, R. S. (2002) Identification of RTG2 as a modifier gene for CTG*CAG repeat instability in Saccharomyces cerevisiae. Genetics. 162, 579–589.
Birrell G. W., Giaever G, Chu A. M., Davis R. W., and Brown, J. M. (2001) A genome-wide screen in Saccharomyces cerevisiae for genes affecting UV radiation sensitivity. Proc. Natl. Acad. Sci. USA 98, 12608–12613.
Bradley J. L., Blake J. C., Chamberlain S, et al. (2000) Clinical, biochemical and molecular genetic correlations in Friedreich’s ataxia. Hum. Mol. Genet. 9, 275–282.
Campuzano V., Montermini L., Molto M. D., et al. (1996) Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271, 1423–1427.
Cavadini P., Gellera C., Patel P. I., and Isaya, G. (2000) Human frataxin maintains mitochondrial iron homeostasis in Saccharomyces cerevisiae. Hum. Mol. Genet. 9:2523–2530.
Chan T. F., Carvalho J., Riles L., and Zheng, X. F. (2000) A chemical genomics approach toward understanding the global functions of the target of rapamycin protein (TOR). Proc. Natl. Acad. Sci. USA 97, 13227–13232.
Chernoff Y.O., Uptain S. M., and Lindquist S. L. (2002) Analysis of prion factors in yeast. Methods Enzymol. 351, 499–538.
Chernoff Y. O. (2001) Mutation processes at the protein level: is Lamarck back? Mutat. Res. 488, 39–64.
Clow A., Greenhalf W., and Chaudhuri, B. (1998) Under respiratory growth conditions, Bcl-x(L) and Bcl-2 are unable to overcome yeast cell death triggered by a mutant Bax protein lacking the membrane anchor. Eur. J. Biochem. 258, 19–28.
Conway K. A., Lee S. J., Rochet J. C., et al. (2000) Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc. Natl. Acad. Sci. USA 97, 571–576.
Cooper A. J., Sheu K. F., Burke J. R., et al. (1997) Polyglutamine domains are substrates of tissue transglutaminase: does transglutaminase play a role in expanded CAG/poly-Q neurodegenerative diseases? J. Neurochem. 69, 431–434.
DePace A. H., Santoso, A., Hillner P., and Weissman, J. S. (1998) A critical role for amino-terminal glutamine/asparagine repeats in the formation and propagation of a yeast prion. Cell 93, 1241–1252.
Derkatch I. L., Bradley M. E., Hong J. Y., and Liebman, S. W. (2001) Prions affect the appearance of other prions: the story of [PIN+]. Cell 106, 171–182.
Derkatch I. L., Bradley M. E., Zhou P, Chernoff Y. O., and Liebman, S. W. (1997) Genetic and environmental factors affecting the de novo appearance of the [PSI+] prion in Saccharomyces cerevisiae. Genetics 147, 507–519.
Eigen, M. (1996) Prionics or the kinetic basis of prion diseases. Biophys. Chem. 63, A1-A18.
Fabunmi R. P., Wigley W. C., Thomas P. J., and DeMartino, G. N. (2000) Activity and regulation of the centrosome-associated proteasome. J. Biol. Chem. 275, 409–413.
Feldman D. E. and Frydman J. (2000) Protein folding in vivo: the importance of molecular chaperones. Curr. Opin. Struct. Biol. 10, 26–33.
Foury F. and Cazzalini O. (1997) Deletion of the yeast homologue of the human gene associated with Friedreich’s ataxia elicits iron accumulation in mitochondria. FEBS Lett. 411, 373–377.
Frohlich K. U. and Madeo, F. (2001) Apoptosis in yeast: a new model for aging research. Exp. Gerontol. 37, 27–31.
Frydman, J. (2001) Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu. Rev. Biochem. 70, 603–647.
Garcia-Mata R, Bebok, Z., Sorscher E. J., and Sztul E. S. (1999) Characterization and dynamics of aggresome formation by a cytosolic GFP-chimera. J. Cell Biol. 146, 1239–1254.
Gardiner, R. M. (1993) Genetic analysis of Batten disease. J. Inherit. Metab. Dis. 16, 787–790.
Giaever G., Chu A.M., Ni L., et al. (2002) Functional profiling of the Saccharomyces cerevisiae genome. Nature 418, 387–391.
Glickman M. H. and Ciechanover A. (2002) The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 82, 373–428.
Goedert, M. (2001) The significance of tau and alpha-synuclein inclusions in neurodegenerative diseases. Curr. Opin. Genet. Dev. 11, 343–351.
Golabek A. A., Kida, E., Walus M., et al. (2000) CLN3 protein regulates lysosomal pH and alters intracellular processing of Alzheimer’s amyloid-beta protein precursor and cathepsin D in human cells. Mol. Genet. Metab. 70, 203–213.
Greenhalf W., Stephan C., and Chaudhuri B. (1996) Role of mitochondria and C-terminal membrane anchor of Bcl-2 in Bax induced growth arrest and mortality in Saccharomyces cerevisiae. FEBS Lett. 380, 169–175.
Gu Y, Verghese S., Mishra R. S., et al. (2003) Mutant prion protein-mediated aggregation of normal prion protein in the endoplasmic reticulum: implications for prion propagation and neurotoxicity. J. Neurochem. 84, 10–22.
Guarente L. (2001) SIR2 and aging—the exception that proves the rule. Trends Genet. 17, 391–392.
Guarente L. and Kenyon C. (2000) Genetic pathways that regulate ageing in model organisms. Nature 408, 255–262.
Hall N. A., Lake B. D., and Patrick A. D. (1991) Recent biochemical and genetic advances in our understanding of Batten’s disease (ceroid-lipofuscinosis). Dev. Neurosci. 13, 339–344.
Haskell R. E., Carr C. J., Pearce D. A., Bennett M. J., and Davidson B. L. (2000) Batten disease: evaluation of CLN3 mutations on protein localization and function. Hum. Mol. Genet. 9, 735–744.
Hattula K. and Peranen J. (2000) FIP-2, a coiled-coil protein, links Huntingtin to Rab8 and modulates cellular morphogenesis. Curr. Biol. 10, 1603–1606.
Hershko A. and Ciechanover A. (1998) The ubiquitin system (review). Annu. Rev. Biochem. 67, 425–479.
Igarashi S., Koide R., Shimohata T., et al. (1998) Suppression of aggregate formation and apoptosis by transglutaminase inhibitors in cells expressing truncated DRPLA protein with an expanded polyglutamine stretch. Nat. Genet. 18, 111–117.
Ireland M. J., Reinke S. S., and Livingston D. M. (2000) The impact of lagging strand replication mutations on the stability of CAG repeat tracts in yeast. Genetics 155, 1657–165.
Jakupciak J. P. and Wells R. D. (2000) Genetic instabilities of triplet repeat sequences by recombination. IUBMB Life 50, 355–359.
James C., Gschmeissner S., Fraser A., and Evan G. I. (1997) CED-4 induces chromatin condensation in Schizosaccharomyces pombe and is inhibited by direct physical association with CED-9. Curr. Biol. 7, 246–252.
Johnston J. A., Illing M. E., and Kopito R. R. (2002) Cytoplasmic dynein/dynactin mediates the assembly of aggresomes. Cell Motil. Cytoskeleton 53, 26–38.
Johnston J. A., Ward C. L., and Kopito R. R. (1998) Aggresomes: a cellular response to misfolded proteins. J. Cell Biol. 143, 1883–1898.
Kaeberlein M., McVey M., and Guarente L. (1999) The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 13, 2570–2580.
Kahlem P., Green H., and Djian P. (1998) Transglutaminase as the agent of neurodegenerative diseases due to polyglutamine expansion. Pathologie Biologie 46, 681–682.
Kang J. J., Schaber M. D., Srinivasula S. M., et al. (1999) Cascades of mammalian caspase activation in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 274, 3189–3198.
Kaufman R. J., Scheuner, D., Schroder, M., et al. (2002) The unfolded protein response in nutrient sensing and differentiation. Nat. Rev. Mol. Cell Biol. 3, 411–421.
Knight S. A., Kim R., Pain D., and Dancis A. (1999) The yeast connection to Friedreich ataxia. Am. J. Hum. Genet. 64, 365–371.
Koutnikova H., Campuzano, V., Foury, F., et al. (1997) Studies of human, mouse and yeast homologues indicate a mitochondrial function for frataxin. Nat. Genet. 16, 345–51.
Krobitsch S. and Lindquist S. (2000) Aggregation of huntingtin in yeast varies with the length of the polyglutamine expansion and the expression of chaperone proteins. Proc. Natl. Acad. Sci. USA 97, 1589–1594.
Lathrop R. H., Casale M., Tobias D. J., Marsh J. L., and Thompson L. M. (1998) Modeling protein homopolymeric repeats: possible polyglutamine structural motifs for Huntington’s disease. Ismb. 6, 105–114.
Lee M. G. and Nurse P. (1987) Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 327, 31–35.
Lehmann S., Milhavet O., and Mange A. (1999) Trafficking of the cellular isoform of the prion protein. Biomed. Pharmacother. 53, 39–46.
Ligr M., Madeo F., Frohlich E., et al. (1998) Mammalian Bax triggers apoptotic changes in yeast. FEBS Lett. 438, 61–65.
Lin S. J., Defossez P. A., and Guarente L. (2000) Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289, 2126–2128.
Lindquist S. (1997) Mad cows meet psi-chotic yeast: the expansion of the prion hypothesis. Cell. 89, 495–498.
Ma Y. and Hendershot L. M. (2002) The mammalian endoplasmic reticulum as a sensor for cellular stress. Cell Stress Chaperones. 7, 222–229.
Ma J. and Lindquist S. (2002) Conversion of PrP to a self-perpetuating PrPSc-like conformation in the cytosol. Science 298, 1785–1788.
Ma J., Wollmann R., and Lindquist S. (2002) Neurotoxicity and neurodegeneration when PrP accumulates in the cytosol. Science 298, 1781–1785.
Ma Y. and Hendershot L. M. (2001) The unfolding tale of the unfolded protein response. Cell. 107, 827–830.
Ma J. and Lindquist S. (2001) Wild-type PrP and a mutant associated with prion disease are subject to retrograde transport and proteasome degradation. Proc. Natl. Acad. Sci. USA 98, 14955–14960.
Ma J. and Lindquist S. (1999) De novo generation of a PrPSc-like conformation in living cells. Nat. Cell Biol. 1, 358–361.
Madeo F., Engelhardt S., Herker E., et al. (2002a) Apoptosis in yeast: a new model system with applications in cell biology and medicine. Curr. Genet. 41, 208–216.
Madeo F., Herker E., Maldener C., et al. (2002b) A caspase-related protease regulates apoptosis in yeast. Mol. Cell. 9, 911–917.
Madeo F., Frohlich E., Ligr M., et al. (1999) Oxygen stress: a regulator of apoptosis in yeast. J. Cell Biol. 145, 757–767.
Madeo F., Frohlich E., and Frohlich K. U. (1997) A yeast mutant showing diagnostic markers of early and late apoptosis. J. Cell Biol. 139, 729–734.
Mathew A., Shi Y., Jolly C., and Morimoto R. I. (2000) Analysis of the mammalian heat-shock response. Inducible gene expression and heat-shock factor activity. Methods Mol. Biol. 99, 217–55.
McLean P. J., Kawamata H., and Hyman B. T. (2001) Alpha-synuclein-enhanced green fluorescent protein fusion proteins form proteasome sensitive inclusions in primary neurons. Neuroscience. 104, 901–912.
McVey M., Kaeberlein M., Tissenbaum H. A., and Guarente L. (2001) The short life span of Saccharomyces cerevisiae sgs1 and srs2 mutants is a composite of normal aging processes and mitotic arrest due to defective recombination. Genetics. 157, 1531–1542.
Meriin A. B., Zhang X., Miliaras N. B., et al. (2003) Aggregation of a polypeptide with expanded polyglutamine domain in yeast cells leads to defects in endocytosis, submitted for publication.
Meriin A. B., Zhang X., He, X., et al. (2002) Huntington toxicity in yeast model depends on polyglutamine aggregation mediated by a prion-like protein Rnq1. J. Cell Biol. 157, 997–1004.
Metzler M., Legendre-Guillemin V., Gan L., et al. (2001) HIP1 functions in clathrin-mediated endocytosis through binding to clathrin and adaptor protein 2. J. Biol. Chem.. 276, 39271–39276.
Michelitsch M. D. and Weissman J. S. (2000) A census of glutamine/asparagine-rich regions: implications for their conserved function and the prediction of novel prions. Proc. Natl. Acad. Sci. USA 97, 11910–11915.
Muchowski P. J. (2002) Protein misfolding, amyloid formation, and neurodegeneration: a critical role for molecular chaperones? Neuron. 35, 9–12.
Muchowski P.J., Ning K., D’Souza-Schorey C., and Fields S. (2002) Requirement of an intact microtubule cytoskeleton for aggregation and inclusion body formation by a mutant huntingtin fragment. Proc. Natl. Acad. Sci. USA 99, 727–732.
Muchowski P. J., Schaffar G., Sittler A., et al. (2000) Hsp70 and Hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc. Natl. Acad. Sci. USA 97, 7841–7846.
Nucifora F. C. Jr., Sasaki M., Peters M. F., et al. (2001) Interference by huntingtin and atrophin-1 with cbp-mediated transcription leading to cellular toxicity. Science 291, 2423–2428.
Pardo, C.A., Rabin B. A., Palmer D. N., and Price D. L. (1994) Accumulation of the adenosine triphosphate synthase subunit C in the mnd mutant mouse. A model for neuronal ceroid lipofuscinosis. Am. J. Pathol. 144, 829–835.
Pearce D. A., Carr C. J., Das B., and Sherman F. (1999a) Phenotypic reversal of the btn1 defects in yeast by chloroquine: a yeast model for Batten disease. Proc. Natl. Acad. Sci. USA 96, 11341–11345.
Pearce D. A., Ferea T., Nosel S. A., Das B., and Sherman F. (1999b) Action of BTN1, the yeast orthologue of the gene mutated in Batten disease. Nat. Genet. 22, 55–58.
Pearce D. A., Nosel S. A., and Sherman F. (1999c) Studies of pH regulation by Btn1p, the yeast homolog of human Cln3p. Mol. Genet. Metab. 66, 320–323.
Pearce D. A. and Sherman F. (1999) Investigation of Batten disease with the yeast Saccharomyces cerevisiae. Mol. Genet. Metab. 66, 314–319.
Pearce D. A. and Sherman F. (1998) A yeast model for the study of Batten disease. Proc. Natl. Acad. Sci. USA 95, 6915–6918.
Pearce D. A. and Sherman F. (1997) BTN1, a yeast gene corresponding to the human gene responsible for Batten’s disease, is not essential for viability, mitochondrial function, or degradation of mitochondrial ATP synthase. Yeast 13, 691–697.
Pirkkala L., Nykanen P., and Sistonen L. (2001) Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 15, 1118–1131.
Puccio H. and Koenig M. (2000) Recent advances in the molecular pathogenesis of Friedreich ataxia. Hum. Mol. Genet. 9, 887–892.
Rao D. S., Chang J. C., Kumar P. D., et al. (2001) Huntingtin interacting protein 1 Is a clathrin coat binding protein required for differentiation of late spermatogenic progenitors. Mol. Cell Biol. 21, 7796–7806.
Richard G. F., Goellner G. M., McMurray C. T., and Haber J. E. (2000) Recombination-induced CAG trinucleotide repeat expansions in yeast involve the MRE11-RAD50-XRS2 complex. EMBO J. 19, 2381–2390.
Richard G. F., Dujon B., and Haber J. E. (1999) Double-strand break repair can lead to high frequencies of deletions within short CAG/CTG trinucleotide repeats. Mol. Gen. Genet. 261, 871–882.
Schulz J. B., Lindenau J., Seyfried J., and Dichgans J. (2000) Glutathione, oxidative stress and neurodegeneration. Eur. J. Biochem. 267, 4904–4911.
Schwartz A. L. and Ciechanover A. (1999) The ubiquitin-proteasome pathway and pathogenesis of human diseases. (review). Annu. Rev. Med. 50, 57–74.
Schweitzer J. K. and Livingston D. M. (1998) Expansions of CAG repeat tracts are frequent in a yeast mutant defective in Okazaki fragment maturation. Hum. Mol. Genet. 7, 69–74.
Severin F. F. and Hyman A. A. (2002) Pheromone induces programmed cell death in S. cerevisiae. Curr. Biol. 12, R233-R235.
Sherman M. Y. and Goldberg A. L. (2001) Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron. 29, 15–32.
Singaraja R. R., Hadano S., Metzler M., et al. (2002) HIP14, a novel ankyrin domain-containing protein, links huntingtin to intracellular trafficking and endocytosis. Hum. Mol. Genet. 11, 2815–2828.
Sittler A., Walter, S., Wedemeyer, N., et al. (1998) SH3GL3 associates with the Huntingtin exon 1 protein and promotes the formation of polygln-containing protein aggregates. Mol Cell. 2, 427–436.
Sondheimer N., Lopez N., Craig E. A., and Lindquist S. (2001) The role of Sis1 in the maintenance of the [RNQ+] prion. EMBO J. 20, 2435–2442.
Sondheimer N. and Lindquist S. (2000) Rnq1: an epigenetic modifier of protein function in yeast. Mol. Cell. 5, 163–172.
Steffan J. S., Kazantsev A., Spasic-Boskovic O., et al. (2000) The Huntington’s disease protein interacts with p53 and CREB-binding protein and represses transcription. Proc. Natl. Acad. Sci. USA 97, 6763–6768.
Steinmetz L. M., Scharfe C., Deutschbauer A. M., et al. (2002) Systematic screen for human disease genes in yeast. Nat. Genet. 31, 400–404.
Takekawa M., Maeda T., and Saito, H. (1998) Protein phosphatase 2Calpha inhibits the human stress-responsive p38 and JNK MAPK pathways. EMBO J. 17, 4744–4752.
Telenius H., Kremer B., Goldberg Y. P., et al. (1994) Somatic and gonadal mosaicism of the Huntington disease gene Cag repeat in brain and sperm. Nat. Genet. 6, 409–414.
Tissenbaum H. A. and Guarente L. (2001) Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 410, 227–230.
Velier J., Kim M., Schwarz C., et al. (1998) Wild-type and mutant huntingtins function in vesicle trafficking in the secretory and endocytic pathways. Exp. Neurol. 152, 34–40.
Vidair C. A., Huang R. N., and Doxsey S. J. (1999) Heat shock causes protein aggregation and reduced protein solubility at the centrosome and other cytoplasmic locations. Int. J. Hyperthermia. 12, 681–695.
Wickner R. B., Taylor K. L., Edskes H. K., et al. (2001) Yeast prions act as genes composed of self-propagating protein amyloids. Adv. Protein Chem. 57, 313–334.
Wigley W. C., Fabunmi R. P., Lee M. G., et al. (1999) Dynamic association of proteasomal machinery with the centrosome. J. Cell Biol. 145, 481–490.
Wilson R. B. and Roof D. M. (1997) Respiratory deficiency due to loss of mitochondrial DNA in yeast lacking the frataxin homologue. Nat. Genet. 16, 352–357.
Winzeler E. A., Shoemaker D. D., Astromoff A., et al. (1999) Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285, 901–906.
Wojcik C., Schroeter D., Wilk S., Lamprecht J., and Paweletz N. (1996) Ubiquitin-mediated proteolysis centers in HeLa cells—indication from studies of an inhibitor of the chymotrypsin-like activity of the proteasome. Eur. J. Cell Biol. 71, 311–318.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sherman, M.Y., Muchowski, P.J. Making yeast tremble. Neuromol Med 4, 133–146 (2003). https://doi.org/10.1385/NMM:4:1-2:133
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/NMM:4:1-2:133