Abstract
Introduction
The Merci Retrieval System® was cleared for use in patients with stroke in August 2004. However, there are few published results of “real world experience” with the device.
Methods
We captured single-center data on 25 consecutive patients with acute ischemic stroke treated with the Merci Retrieval System according to the MERCI trial except that we treated some patients with tandem proximal carotid and intracranial lesions with carotid angioplasty and stenting and some patients were treated within the 3-hour window.
Results
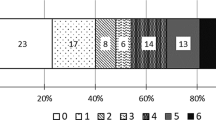
Median patient age was 63 years and median initial National Institute of Health Stroke Scale (NIHSS) score was 18. Isolated M1 or M2 middle cerebral artery lesions occurred in 52% “carotid T” lesions in 8%, and vertebrobasilar lesions in 8% Tandem lesions involving proximal carotid and proximal intracranial vessel occurred in 32%, necessitating emergent multilevel treatment including carotid stenting. Median duration from symptom onset to Merci device utilization was 5.2 hours. Successful reperfusion (≥thrombolysis in myocardial infarction [TIMI] 2 flow) in the target vessel was obtained in 56% of cases. Statistical analysis revealed a strong correlation between ability to achieve greater than or equal to TIMI 2 flow a good clinical outcome as measured by 3-month NIHSS score, modified Rankin Scale (mRS), and mortality (nine out of the 12 without successful reperfusion died compared to none of the 13 with ≥TIMI 2 flow, p<0.001). Younger age and lower NIHSS score on presentation were also predictors of good clinical outcome at 3 months.
Conclusion
These “real world data” demonstrate that the results of the previous MERCI trial can be “independently replicated” at a regional stroke center. Although the results of placebo-controlled trials are still pending, mechanical revascularization has become a critical component of our acute stroke protocol, particularly for severe strokes. Issues still remain regarding recalcitrant lesions and operator experience, which necessitate further clinical testing and device optimization.
Similar content being viewed by others
References
The National Institute of Neurological Disorders and Stroke t-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587.
Tanne D, Bates VE, Vero P, et al. Initial clinical experience with IV tissue plasminogen activator for acute ischemic stroke: a multicenter survey. The t-PA Stroke Survey Group. Neurology 1999;53:424–427.
Nilasena DS, Kresowik TE, Wilburn RT, et al. Assessing patterns of t-PA use in acute stroke. Stroke 2002;33:354.
Clark WM, Wissman S, Albers GW, et al. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial: Atleplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Strokes. JAMA 1999;282:2018–2026.
Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase or acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism JAMA 1999;282:2003–2011.
IMS trial investigators. Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the interventional management of stroke study. Stroke 2004;35:904–911.
Schneck MJ, Biller J. New treatments in acute ischemic stroke. Curr Treat Options Neurol 2005;7:499–511.
US Food and Drug Administration. FDA Neurological Devices Panel Fifteenth Meeting. Available at: http://wwwfda.gov/ohrms/ dockets/ac/05/minutes/2004-4083m1_summary%20minutes.doc. Accessed November 6, 2006.
Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 2005;36:1432–1438.
Smith WS. The results of the Multi MERCI trial. Stroke 2006;37:711–712.
National Institutes of Health. Clinical Trials. Protocol Number: NCT0094588. Available at: http://www.clinicaltrials.gov/ct/ show/NCT00094588?order=1.Accessed November 6, 2006.
Broderick J. The IMS III trial: a comparison of a combined IV/IA approach to recanalization with standard IV rtPA within 3 hours of onset. International Stroke Conference 2006, Feb 17, 2006. Kissimmee, FL.
Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke: the European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274:1017–1025. Abstract.
Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind phacebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet 1998; 352:1245–1251.
Damjanoy I, Linder J, eds. Anderson’s Pathology, 10th Edition. St. Louis: Mosby, 1996, pp. 477–483.
Qureshi AI, Huston AD, Harbaugh RE, Stieg PE, Hopkins LN; North American Trial of Unruptured and Ruptured Aneurysms Planning Committee. Methods and design considerations for randomized clinical trials evaluating surgical or endovascular treatments for cerebrovascular diseases. Neurosurgery 2004;54:248–264.
Brandt T, von Kummer R, Muller-Kuppers M, Hacke W. Thrombolytic therapy of acute basilar artery occlusion: variables affecting recanalization and outcome. Stroke 1996;27:875–881.
Hacke W, Zeumer H, Ferbert A, Bruckmann H, del Zoppo GJ. Intra-arterial thrombolytic therapy improves outcome, in patients with acute vertebrobasilar occlusive disease. Stroke 1998;19:1216–1222.
Molina C: Degree of arterial recanalization: an endopoint for efficacy in future intravenous thrombolytic trials. Stroke 2004;35:114.
Molina C, Montaner J, Abilleira S, et al. Speed of intracranial clot lysis with intravenous tissue plasminogen activator therapy: sonographic classification and short-term improvement. Circulation 2001;103:2897.
Felberg R, Okon N, El-Mitwalli A, et al. Early dramatic recovery during intravenous tissue plasminogen activator infusion: clinical pattern and outcome in acute middle cerebral artery stroke. Stroke 2002;33:1301.
Parsons M, Barber P, Chalk J, et al. Diffusion and perfusion-weighted MRI response to thrombolysis in stroke. Ann Neurol 2005;51:28
Wolpert S, Bruckmann H, Greenlee R, et al. Neuroradiologic evaluation of patidnts with acute stroke treated with recombinant tissue plasminogen activator. AJNR 1993;42:976–982
Yamaguchi T, Hayakawa T, Kiuchi H, For the Japanese Thrombolysis Study Group. Intravenous tissue plasminogen activator ameliorates the outcome of hyperacute embolic stroke. Cerebrovasc Dis 1993;3:269–272.
Mori E, Yoneda Y, Tabuchi M, et al. Intravenous recombinant tissue plasminogen activator in acute carotid artery territory stroke. Neurology 1992;42:976–982.
Lewandwoski CA, Frankel M, Tomsick TA, et al. Combined intravenous and intra-arterial r-tPA versus intra-arterial therapy of acute ischemic stroke: Emergency Management of Stroke (EMS) Bridging Trial. Stroke 1999;30(12):2598–2605.
Clinical highlights from the NINDS t-PA Stroke Trial. San Francisco (CA): Genentech, Inc., 1997, p. 27. Report No.:G71121-RO LB03.
Dunn EJ, Philippou H, Ariens RA, Grant PJ. Molecular mechanisms involved in the resistance of fibrin to clot lysis by plasmin in subjects with type 2 diabetes mellitus. Diabetologia 2006; 49(5):1071–1080.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Devlin, T.G., Baxter, B.W., Feintuch, T.A. et al. The merci retrieval system for acute stroke. Neurocrit Care 6, 11–21 (2007). https://doi.org/10.1385/NCC:6:1:11
Issue Date:
DOI: https://doi.org/10.1385/NCC:6:1:11