Abstract
Introduction: Fundamental principles in the central nervous system are that primary depolarization of neurons causes hyperemia, whereas focal cerebral ischemia causes secondary neuronal depolarization. In rats, an exception to these rules was discovered recently in which primary neuronal depolarization led to focal cerebral ischemia via inverse coupling between neuronal metabolism and cerebral blood flow (CBF). Adenosine is one of the classical candidate factors to mediate the coupling between neuronal metabolism and CBF. Therefore, the effect of topically applied adenosine on cortical spreading ischemia was studied.
Methods: A cranial window was implanted in 10 rats. At the window, CBF (laser Doppler flowmetry) and the subarachnoid direct current potential were recorded; the cortical surface was superfused with artificial cerebrospinal fluid (ACSF). A spreading neuronal/astroglial depolarization wave was triggered at a remote site, from which it propagated to the cranial window.
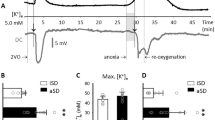
Results: In all rats, the depolarization wave triggered a hyperemic event under physiological conditions. When ACSF containing the nitric oxide (NO)-synthase inhibitor NG-nitro-l-arginine (l-NNA) at 10−3M and K+ at 20×10−3M was subsequently superfused, the depolarization wave triggered an ischemic event. In 5 of 10 animals, a second depolarization wave under l-NNA and elevated K+ also triggered an ischemic event. In contrast, in the remaining five animals, the depolarization wave triggered a significantly smaller and shorter hypoperfusion when adenosine at 100 µM was coapplied with l-NNA and elevated K+.
Conclusion: The results of this study suggest that adenosine, like other vasodilators, is unable to antagonize the initial hypoperfusion in response to a spreading neuronal/astroglial depolarization wave when the NO concentration is reduced and K+ is elevated but shortens the hypoperfusion phase significantly.
Similar content being viewed by others
References
Villringer A, Dirnagl U. Coupling of brain activity and cerebral blood flow: basis of functional neuroimaging. Cerebrovasc Brain Metab Rev 1995;7:240–276.
Leão AAP. Spreading depression of activity in the cerebral cortex. J Neurophysiol 1944;7:359–390.
Dreier JP, Körner K, Ebert N, et al. Nitric oxide scavenging by haemoglobin or nitric oxide synthase inhibition by N-nitro-L-arginine induce cortical spreading ischemia when K+ is increased in the subarachnoid space. J Cereb Blood Flow Metab 1998;18:978–990.
Dreier JP, Ebert N, Priller J, et al. Products of hemolysis in the subarachnoid space induce cortical spreading ischemia and focal necrosis in rats: a model for delayed ischemic neurological deficits after subarachnoid haemorrhage? J Neurosurg 2000;93:668–676.
Dreier JP, Windmüller O, Petzold G, Lindauer U, Einhäupl KM, Dirnagl U. Ischemia triggered by red blood cell products in the subarachnoid space is inhibited by nimodipine or moderate volume expansion/hemodilution in rats. Neurosurgery 2002;51:1457–1467.
Dreier JP, Petzold G, Tille K, et al. Ischaemia triggered by spreading neuronal activation is inhibited by vasodilators in rats. J Physiol (Lond) 2001;531:515–526.
Feigin VL, Rinkel GJ, Algra A, Vermeulen M, van Gijn J. Calcium antagonists in patients with aneurysmal subarachnoid hemorrhage: a systematic review. Neurology 1998;50:876–883.
Van Gijn J, Rinkel GJE. Subarachnoid haemorrhage: diagnosis, causes and management. Brain 2001;124:249–278.
Hasan D, Vermeulen M, Wijdicks EF, Hijdra A, van Gijn J. Effect of fluid intake and antihypertensive treatment on cerebral ischemia after subarachnoid hemorrhage. Stroke 1989;20:1511–1515.
Vermeij FH, Hasan D, Bijvoet HW, Avezaat CJ. Impact of medical treatment on the outcome of patients after aneurismal subarachnoid hemorrhage. Stroke 1998;29:924–930.
Rosenwasser RH, Delgado TE, Buchheit WA, Freed MH. Control of hypertension and prophylaxis against vasospasm in cases of subarachnoid hemorrhage: a preliminary report. Neurosurgery 1983;12:658–661.
Morii S, Ngai AC, Ko KR, Winn HR. Role of adenosine in regulation of cerebral blood flow: effects of theophylline during normoxia and hypoxia. Am J Physiol 1987;253:H165-H175.
Winn HR, Rubio GR, Berne RM. The role of adenosine in the regulation of cerebral blood flow. J Cereb Blood Flow Metab 1981;1:239–244.
Kaku T, Hada J, Hayashi Y. Endogenous adenosine exerts inhibitory effects upon the development of spreading depression and glutamate release induced by microdialysis with high K+ in rat hippocampus. Brain Res 1994;658:39–48.
Ko KR, Ngai AC, Winn HR. Role of adenosine in regulation of regional cerebral blood flow in sensory cortex. Am J Physiol 1990;259:H1703-H1708.
Barfod C, Akgören N, Fabricius M, Dirnagl U, Lauritzen M. Laser-Doppler measurements of concentration and velocity of moving blood cells in rat cerebral circulation. Acta Physiol Scand 1997;160:123–132.
Edvinsson L, Fredholm BB. Characterization of adenosine receptors in isolated cerebral arteries of cat. Br J Pharmacol 1983;80:631–637.
Fenton RA, Bruttig SP, Rubio R, Berne RM. Effect of adenosine on calcium uptake by intact and cultured vascular smooth muscle. Am J Physiol 1982;242:H797-H804.
Foley DH. Diminished arterial smooth muscle response to adenosine during Na-K pump inhibition. Pflugers Arch 1984;400:88–95.
Dirnagl U, Niwa K, Lindauer U, Villringer A. Coupling of cerebral blood flow to neuronal activation: role of adenosine and nitric oxide. Am J Physiol 1994;267:H296-H301.
Armstead WM. Role of nitric oxide, cyclic nucleotides, and the activation of ATP-sensitive K+ channels in the contribution of adenosine to hypoxia-induced pial artery dilation. J Cereb Blood Flow Metab 1997;17:100–108.
Kuo L, Chancellor JD. Adenosine potentiates flow-induced dilation of coronary arterioles by activating KATP channels in endothelium. Am J Physiol 1995;269:H541-H549.
Vials A, Burnstock G. A2-purinoceptor-mediated relaxation in the guinea-pig coronary vasculature: a role for nitric oxide. Br J Pharmacol 1993;109:424–429.
Ngai AC, Winn HR. Modulation of cerebral arteriolar diameter by intraluminal flow and pressure. Circ Res 1995;77:832–840.
Colonna DM, Meng W, Deal DD, Busija DW. Nitric oxide promotes arteriolar dilation during cortical spreading depression in rabbits. Stroke 1994;25:2463–2470.
Wei EP, Kukreja R, Kontos HA. Effects in cats of inhibition of nitric oxide synthesis on cerebral vasodilation and endothelium-derived relaxing factor from acetylcholine. Stroke 1992;23:1623–1628.
Conde MV, Marco EJ, Fraile ML, et al. Different influence of endothelium in the mechanical responses of human and cat isolated cerebral arteries to several agents. J Pharm Pharmacol 1991;43:255–261.
Haberl RL, Anneser F, Villringer A, Einhaupl KM. Angiotensin II induces endothelium-dependent vasodilation of rat cerebral arterioles. Am J Physiol 1990;258:H1840-H1846.
Kraig RP, Nicholson C. Extracellular ionic variations during spreading depression. Neuroscience 1978;3:1045–1059.
Nishiye E, Nakao K, Itoh T, Kuriyama H. Factors inducing endothelium-dependent relaxation in the guinea-pig basilar artery as estimated from the actions of haemoglobin. Br J Pharmacol 1989;96:645–655.
Minato H, Hashizume M, Masuda Y, Hosoki K. Modulation of extraluminally induced vasoconstrictions by endothelium-derived nitric oxide in the canine basilar artery. Arzneimittelforschung 1995;45:675–678.
Golding EM, Steenberg ML, Johnson TD, Bryan RM. Nitric oxide in the potassium-induced response of the rat middle cerebral artery: a possible permissive role. Brain Res 2001;889:98–104.
Schuh-Hofer S, Lobsien E, Brodowsky R, et al. The cerebrovascular response to elevated potassium-role of nitric oxide in the in vitro model of isolated rat middle cerebral arteries. Neurosci Lett 2001;306:61–64.
Duckrow RB. A brief hypoperfusion precedes spreading depression if nitric oxide synthesis is inhibited. Brain Res 1993;618:190–195.
Hajek I, Subbarao KV, Hertz L. Acute and chronic effects of potassium and noradrenaline on Na+, K+-ATPase activity in cultured mouse neurons and astrocytes. Neurochem Int 1996;28:335–342.
MacDonald RL, Weir BKA. A review of hemoglobin and the pathogenesis of cerebral vasospasm. Stroke 1991;22:971–982.
Edwards DH, Byrne JV, Griffith TM. The effect of chronic subarachnoid hemorrhage on basal endothelium-derived relaxing factor activity in intrathecal cerebral arteries. J Neurosurg 1992;76:830–837.
Pluta RM, Thompson BG, Dawson TM, Snyder SH, Boock RJ, Oldfield EH. Loss of nitric oxide synthase immunoreactivity in cerebral vasospasm. J Neurosurg 1996;84:648–654.
Ohta O, Osaka K, Siguma M, Yamamoto M, Shimizu K, Toda N. Cerebral vasospasm following ruptured intracranial aneurysms, especially some contributions of potassium ion released from subarachnoid hematoma to delayed cerebral vasospasm. In: Bevan JA, ed. Vascular Neuroeffector Mechanisms. New York: Raven, 1983:353–358.
Marzatico F, Gaetani P, Rodriguez y Baena R, et al. Experimental subarachnoid hemorrhage. Lipid peroxidation and Na(+),K(+)-ATPase in different rat brain areas. Mol Chem Neuropathol 1989;11:99–107.
Birse SH, Tom MI. Incidence of cerebral infarction associated with ruptured intracranial aneurysms. A study of 8 unoperated cases of anterior cerebral aneurysm. Neurology 1960;10:101–106.
Stoltenburg-Didinger G, Schwarz K. Brain lesions secondary to subarachnoid hemorrhage due to ruptured aneurysms. In: Cervós-Navarro J, Ferszt R, eds. Stroke and Microcirculation. New York: Raven, 1987:471–480.
Neil-Dwyer G, Lang DA, Doshi B, Gerber CJ, Smith PW. Delayed cerebral ischaemia: the pathological substrate. Acta Neurochir (Wien) 1994;131:137–145.
Dreier JP, Sakowitz OW, Harder A, et al. Focal laminar cortical MR signal abnormalities after subarachnoid hemorrhage. Ann Neurol 2002;52:825–829.
Nosko M, Krueger CA, Weir BK, Cook DA. Effects of nimodipine on in vitro contractility of cerebral arteries of dog, monkey, and man. J Neurosurg 1986;65:376–381.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dreier, J.P., Tille, K. & Dirnagl, U. Partial antagonistic effect of adenosine on inverse coupling between spreading neuronal activation and cerebral blood flow in rats. Neurocrit Care 1, 85–94 (2004). https://doi.org/10.1385/NCC:1:1:85
Issue Date:
DOI: https://doi.org/10.1385/NCC:1:1:85