Abstract
Bipolar disorder is a devastating disease with a lifetime incidence of about 1% in the general population. Suicide is the cause of death in 10 to 15% of patients and in addition to suicide, mood disorders are associated with many other harmful health effects. Mood stabilizers are medications used to treat bipolar disorder. In addition to their therapeutic effects for the treatment of acute manic episodes, mood stabilizers are useful as prophylaxis against future episodes and as adjunctive antidepressant medications. The most established and investigated mood-stabilizing drugs are lithium and valproate but other anticonvulsants (such as carbamazepine and lamotrigine) and antipsychotics are also considered as mood stabilizers. Despite the efficacy of these diverse medications, their mechanisms of action remain, to a great extent, unknown. Lithium’s inhibition of some enzymes, such as inositol monophosphatase and gycogen synthase kinase-3, probably results in its mood-stabilizing effects. Valproate may share its anticonvulsant target with its mood-stabilizing target or may act through other mechanisms. It has been shown that lithium, valproate, and/or carbamazepine regulate numerous factors involved in cell survival pathways, including cyclic adenine monophospate response element-binding protein, brain-derived neurotrophic factor, bcl-2, and mitogen-activated protein kinases. These drugs have been suggested to have neurotrophic and neuroprotective properties that ameliorate impairments of cellular plasticity and resilience underlying the pathophysiology of mood disorders. This article also discusses approaches to develop novel treatments specifically for bipolar disorder.
Similar content being viewed by others
References
Tohen M., Hennen J., Zarate C. M. Jr., et al. (2000) Two-year syndromal and functional recovery in 219 cases of first-episode major affective disorder with psychotic features. Am. J. Psychiatry 157, 220–228.
Benazzi F. (2001) Prevalence and clinical correlates of residual depressive symptoms in bipolar II disorder. Psychother. Psychosom. 70, 232–238.
Keitner G. I., Solomon D. A., Ryan C. E., et al. (1996) Prodromal and residual symptoms in bipolar I disorder. Compr. Psychiatry 37, 362–367.
Gitlin M. J., Swendsen J., Heller T. L., and Hammen C. (1995) Relapse and impairment in bipolar disorder. Am. J. Psychiatry 152, 1635–1640.
Zarate C. A. Jr., Tohen M., Land M., and Cavanagh S. (2000) Functional impairment and cognition in bipolar disorder. Psychiatr. Q. 71, 309–329.
MacQueen G. M., Young L. T., and Joffe R. T. (2001) A review of psychosocial outcome in patients with bipolar disorder. Acta. Psychiatr. Scand. 103, 163–170.
Murray C. J. and Lopez A. D. (1997) Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 349, 1498–1504.
Musselman D. L., Evans D. L., and Nemeroff C. B. (1998) The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch. Gen. Psychiatry 55, 580–592.
Michelson D., Stratakis C., Hill L., et al. (1996) Bone mineral density in women with depression. N. Engl. J. Med. 335, 1176–1181.
Ciechanowski P. S., Katon W. J., and Russo J. E. (2000) Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch. Intern. Med. 160, 3278–3285.
Gould T. D. and Manji H. K. (2002) Signaling networks in the pathophysiology and treatment of mood disorders. J. Psychosom. Res. 53, 687–697.
Manji H. K., Bebchuk J. M., Moore G. J., Glitz D., Hasanat K. A., and Chen G. (1999) Modulation of CNS signal transduction pathways and gene expression by mood-stabilizing agents: therapeutic implications. J. Clin. Psychiatry 60, 27–39; discussion 40–21, 113–116.
Manji H. K., Chen G., Hsiao J. K., Risby E. D., Masana M. I., and Potter W. Z. (1996) Regulation of signal transduction pathways by mood-stabilizing agents: implications for the delayed onset of therapeutic efficacy. J. Clin. Psychiatry 57, 34–46; discussion 47–38.
Chen G., Manji H. K., Hawver D. B., Wright C. B., and Potter W. Z. (1994) Chronic sodium valproate selectively decreases protein kinase C alpha and epsilon in vitro. J. Neurochem. 63, 2361–2364.
Williams R. S., Cheng L., Mudge A. W., and Harwood A. J. (2002) A common mechanism of action for three mood-stabilizing drugs. Nature 417, 292–295.
Lenox R. H., McNamara R. K., Watterson J. M., and Watson D. G. (1996) Myristoylated alanine-rich C kinase substrate (MARCKS): a molecular target for the therapeutic action of mood stabilizers in the brain? J. Clin. Psychiatry 57, 23–31; discussion 32–33.
Lenox R. H. and Wang L. (2003) Molecular basis of lithium action: integration of lithium-responsive signaling and gene expression networks. Mol. Psychiatry 8, 135–144.
Coyle J. T. and Duman R. S. (2003) Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron 38, 157–160.
Harwood A. J. and Agam G. (2003) Search for a common mechanism of mood stabilizers. Biochem. Pharmacol. 66, 179–189.
Post R. M. (2000) Psychopharmacology of mood stabilizers. In: Schizophrenia and Mood Disorders: The New Drug Therapies in Clinical Practice., Buckley P. F. and Waddington J. L., eds. Oxford: Butterworth-Heinemann, pp. 127–154.
Ryves W. J. and Harwood A. J. (2001) Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem. Biophys. Res. Commun. 280, 720–725.
Davies S. P., Reddy H., Caivano M., and Cohen P. (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351, 95–105.
Amari L., Layden B., Rong Q., Geraldes C. F., and Mota de Freitas D. (1999) Comparison of fluorescence, (31)P NMR, and (7)Li NMR spectroscopic methods for investigating Li(+)/Mg(2+) competition for biomolecules. Anal. Biochem. 272, 1–7.
York J. D., Ponder J. W. and Majerus P. W. (1995) Definition of a metal-dependent/Li(+)-inhibited phosphomonoesterase protein family based upon a conserved three-dimensional core structure. Proc. Natl. Acad. Sci. USA 92, 5149–5153.
Masuda C. A., Xavier M. A., Mattos K. A., Galina A., and Montero-Lomeli M. (2001) Phosphoglucomutase is an in vivo lithium target in yeast. J. Biol. Chem. 10, 10.
Kajda P. K. and Birch N. J. (1981) Lithium inhibition of phosphofructokinase. J. Inorg. Biochem. 14, 275–278.
Klein P. S. and Melton D. A. (1996) A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA 93, 8455–8459.
Johannessen C. U. (2000) Mechanisms of action of valproate: a commentatory. Neurochem. Int. 37, 103–110.
van der Laan J. W., de Boer T., and Bruinvels J. (1979) Di-n-propylacetate and GABA degradation. Preferential inhibition of succinic semialdehyde dehydrogenase and indirect inhibition of GABA-transaminase. J. Neurochem. 32, 1769–1780.
Sawaya M. C., Horton R. W., and Meldrum B. S. (1975) Effects of anticonvulsant drugs on the cerebral enzymes metabolizing GABA. Epilepsia 16, 649–655.
Whittle S. R. and Turner A. J. (1978) Effects of the anticonvulsant sodium valproate on gamma-aminobutyrate and aldehyde metabolism in ox brain. J. Neurochem. 31, 1453–1459.
Anlezark G. M., Horton R. W., Meldrum B. S., Sawaya M. C., and Stephenson J. D. (1976) Proceedings: gamma-aminobutyric acid metabolism and the anticonvulsant action of ethanolamine-o-sulphate and di-n-propylacetate. Br. J. Pharmacol. 56, 383P,384P.
Gottlicher M., Minucci S., Zhu P., et al. (2001) Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. Embo. J. 20, 6969–6978.
Tremolizzo L., Carboni G., Ruzicka W. B., et al. (2002) An epigenetic mouse model for molecular and behavioral neuropathologies related to schizophrenia vulnerability. Proc. Natl. Acad. Sci. USA 99, 17,095–17,100.
Yildirim E., Zhang Z., Uz T., Chen C. Q., Manev R., and Manev H. (2003) Valproate administration to mice increases histone acetylation and 5-lipoxygenase content in the hippocampus. Neurosci. Lett. 345, 141–143.
Gould T. D., Chen G., and Manji H. K. (2003) In Vivo Evidence in the Brain for Lithium Inhibition of Glycogen Synthease Kinase-3. In Neuropsychopharmacology.
Bourne H. R. and Nicoll R. (1993) Molecular machines integrate coincident synaptic signals. Cell 72(Suppl), 65–75.
Bhalla U. S. and Iyengar R. (1999) Emergent properties of networks of biological signaling pathways. Science 283, 381–387.
Weng G., Bhalla U. S., and Iyengar R. (1999) Complexity in biological signaling systems. Science 284, 92–96.
Manji H. K. (1992) G proteins: implications for psychiatry. Am. J. Psychiatry 149, 746–760.
Szabo S. T., Gould T. D., and Manji H. K. (2003) Introduction to neurotransmitters, receptors, signal transduction, and second messengers. In: Textbook of Psychopharmacology, Nemeroff C., ed. Arlington, VA: American Psychiatric Publishing, pp. 3–52.
Rasenick M. M., Chaney K. A., and Chen J. (1996) G protein-mediated signal transduction as a target of antidepressant and antibipolar drug action: evidence from model systems. J. Clin. Psychiatry 57, 49–55; discussion 56–58.
Berridge M. J., Downes C. P., and Hanley M. R. (1989) Neural and developmental actions of lithium: a unifying hypothesis. Cell 59, 411–419.
Allison J. H., Blisner M. E., Holland W. H., Hipps P. P., and Sherman W. R. (1976) Increased brain myo-inositol 1-phosphate in lithium-treated rats. Biochem. Biophys. Res. Commun. 71, 664–670.
Allison J. H. and Stewart M. A. (1971) Reduced brain inositol in lithium-treated rats. Nat. New Biol. 233, 267,268.
Hallcher L. M. and Sherman W. R. (1980) The effects of lithium ion and other agents on the activity of myo- inositol-1-phosphatase from bovine brain. J. Biol. Chem. 255, 10,896–10,901.
Naccarato W. F., Ray R. E., and Wells W. W. (1974) Biosynthesis of myo-inositol in rat mammary gland. Isolation and properties of the enzymes. Arch. Biochem. Biophys. 164, 194–201.
Berridge M. J., Downes C. P., and Hanley M. R. (1982) Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem. J. 206, 587–595.
Manji H. K. and Lenox R. H. (1999) Ziskind-Somerfeld Research Award. Protein kinase C signaling in the brain: molecular transduction of mood stabilization in the treatment of manic-depressive illness. Biol. Psychiatry 46, 1328–1351.
Manji H. K., Bersudsky Y., Chen G., Belmaker R. H., and Potter W. Z. (1996) Modulation of protein kinase C isozymes and substrates by lithium: the role of myo-inositol. Neuropsychopharmacology 15, 370–381.
Manji H. K., Etcheberrigaray R., Chen G., and Olds J. L. (1993) Lithium decreases membrane-associated protein kinase C in hippocampus: selectivity for the alpha isozyme. J. Neurochem. 61, 2303–2310.
Leli U. and Hauser G. (1992) Lithium modifies diacylglycerol levels and protein kinase C in neuroblastoma cells. Abstracts of the 8th international conference on second messengers and phosphoproteins, Z187F.
Li X. and Jope R. S. (1995) Selective inhibition of the expression of signal transduction proteins by lithium in nerve growth factor-differentiated PC12 cells. J. Neurochem. 65, 2500–2508.
Lenox R. H., Watson D. G., Patel J., and Ellis J. (1992) Chronic lithium administration alters a prominent PKC substrate in rat hippocampus. Brain. Res. 570, 333–340.
Watson D. G. and Lenox R. H. (1996) Chronic lithium-induced down-regulation of MARCKS in immortalized hippocampal cells: potentiation by muscarinic receptor activation. J. Neurochem. 67, 767–777.
Watson D. G., Watterson J. M., and Lenox R. H. (1998) Sodium valproate down-regulates the myristoylated alanine-rich C kinase substrate (MARCKS) in immortalized hippocampal cells: a property of protein kinase C-mediated mood stabilizers. J. Pharmacol. Exp. Ther. 285, 307–316.
Fibiger H. C. (1995) Neurobiology of depression: focus on dopamine. Adv. Biochem. Psychopharmacol. 49, 1–17.
Goodwin F. K. and Jamison K. R. (1990) Manic-Depressive Illness. New York: Oxford University Press.
Einat H., Yuan P., Gould T. D., et al. (2003) The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J. Neurosci. 23, 7311–7316.
Nestler E. J., Gould E., Manji H., et al. (2002) Preclinical models: status of basic research in depression. Biol. Psychiatry 52, 503–528.
Giambalvo C. T. (1992) Protein kinase C and dopamine transport—2. Effects of amphetamine in vitro. Neuropharmacology 31, 1211–1222.
Gnegy M. E., Hong P., and Ferrell S. T. (1993) Phosphorylation of neuromodulin in rat striatum after acute and repeated, intermittent amphetamine. Brain. Res. Mol. Brain Res. 20, 289–298.
Iwata S., Hewlett G. H., and Gnegy M. E. (1997) Amphetamine increases the phosphorylation of neuromodulin and synapsin I in rat striatal synaptosomes. Synapse 26, 281–291.
Iwata S. I., Hewlett G. H., Ferrell S. T., Kantor L., and Gnegy M. E. (1997) Enhanced dopamine release and phosphorylation of synapsin I and neuromodulin in striatal synaptosomes after repeated amphetamine. J. Pharmacol. Exp. Ther. 283, 1445–1452.
Birnbaum S. G., Yuan P. X., Wang M., et al. (2004) Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science 306, 882–884.
Moore G. J., Bebchuk J. M., Parrish J. K., et al. (1999) Temporal dissociation between lithium-induced changes in frontal lobe myo-inositol and clinical response in manic-depressive illness. Am. J. Psychiatry 156, 1902–1908.
Hahn C. G. and Friedman E. (1999) Abnormalities in protein kinase C signaling and the pathophysiology of bipolar disorder. Bipolar Disord. 1, 81–86.
Friedman E., Hoau Yan W., Levinson D., Connell T. A., and Singh H. (1993) Altered platelet protein kinase C activity in bipolar affective disorder, manic episode. Biol. Psychiatry 33, 520–525.
Wang H. Y. and Friedman E. (1996) Enhanced protein kinase C activity and translocation in bipolar affective disorder brains. Biol. Psychiatry 40, 568–575.
Kao K. R., Masui Y., and Elinson R. P. (1986) Lithium-induced respecification of pattern in Xenopus-laevis embryos. Nature 322, 371–373.
He X., Saint-Jeannet J. P., Woodgett J. R., Varmus H. E., and Dawid I. B. (1995) Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature 374, 617–622.
Klein P. S. and Melton D. A. (1996) A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA 93, 8455–8459.
Stambolic C., Ruel L., and Woodgett J. R. (1996) Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 6, 1664–1668.
Gurvich N. and Klein P. S. (2002) Lithium and valproic acid: parallels and contrasts in diverse signaling contexts. Pharmacol. Ther. 96, 45–66.
O’Brien W. T., Harper A. D., Jove F., et al. (2004) Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J. Neurosci. 24, 6791–6798.
Phiel C. J., Wilson C. A., Lee V. M., and Klein P. S. (2003) GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature 423, 435–439.
Chalecka-Franaszek E. and Chuang D. M. (1999) Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proc. Natl. Acad. Sci. USA 96, 8745–8750.
Zhang F., Phiel C. J., Spece L., Gurvich N., and Klein P. S. (2003) Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3. J. Biol. Chem. 278, 33,067–33,077.
Kirshenboim N., Plotkin B., Shlomo S. B., Kaidanovich-Beilin O., and Eldar-Finkelman H. (2004) Lithium-mediated phosphorylation of glycogen synthase kinase-3b involves PI3 kinase-dependent activation of protein kinase C-alpha. J. Mol. Neurosci. 24, 237–245.
Frame S. and Cohen P. (2001) GSK3 takes centre stage more than 20 years after its discovery. Biochem. J. 359, 1–16.
Gould T. D., Zarate C. A. J., and Manji H. K. Glycogen synthase kinase-3: a target for novel bipolar disorder treatments. J. Clin. Psychiatry, in press.
Woodgett J. R. (2001) Judging a protein by more than its name: gsk-3. STKE 2001, RE12.
Gould T. D and Manji H. K. (2002) The wnt signaling pathway in bipolar disorder. Neuroscientist 8, 497–511.
Lenox R. H., Gould T. D., and Manji H. K. (2002) Endophenotypes in bipolar disorder. Am. J. Med. Genet. 114, 391–406.
Jope R. S. and Bijur G. N. (2002) Mood stabilizers, glycogen synthase kinase-3beta and cell survival. Mol. Psychiatry 7(Suppl 1), S35-S45.
Manji H. K., Moore G. J., and Chen G. (1999) Lithium at 50: have the neuroprotective effects of this unique cation been overlooked? Biol. Psychiatry 46, 929–940.
Kaidanovich-Beilin O., Milman A., Weizman A., Pick C. G., and Eldar-Finkelman H. (2004) Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol. Psychiatry 55, 781–784.
Gould T. D., Einat H., Bhat R., and Manji H. K. (2004) AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int. J. Neuropsychopharmacol. 1–4.
Li X., Zhu W., Roh M. S., Friedman A. B., Rosborough K., and Jope R. S. (2004) In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology 29, 1426–1431.
Beaulieu J. M., Sotnikova T. D., Yao W. D., et al. (2004) Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc. Natl. Acad. Sci. USA 101, 5099–5104.
Phiel C. J. and Klein P. S. (2001) Molecular targets of lithium action. Annu. Rev. Pharmacol. Toxicol. 41, 789–813.
Phiel C. J., Zhang F., Huang E. Y., Guenther M. G., Lazar M. A., and Klein P. S. (2001) Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 276, 36,734–36,741.
Chen G., Huang L. D., Jiang Y. M., and Manji H. K. (1999) The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J. Neurochem. 72, 1327–1330.
Hall A. C., Brennan A., Goold R. G., et al. (2002) Valproate Regulates GSK-3-Mediated Axonal Remodeling and Synapsin I Clustering in Developing Neurons. Mol. Cell Neurosci. 20, 257–270.
Li X., Bijur G. N., and Jope R. S. (2002) Glycogen synthase kinase 3-beta, mood stabilizers, and neuroprotection. Bipolar Disorders 4, 137–144.
Grimes A. C. and Jope R. S. (2001) CREB DNA binding activity is inhibited by glycogen synthase kinase-3beta and facilitated by lithium. J. Neurochem. 78, 1–15.
Sheline Y. I. (2003) Neuroimaging studies of mood disorder effects on the brain. Biol. Psychiatry 54, 338–352.
Drevets W. C. (2000) Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog. Brain. Res. 126, 413–431.
Manji H. K., Drevets W. C., and Charney D. S. (2001) The cellular neurobiology of depression. Nat. Med. 7, 541–547.
Drevets W. C. (2003) Neuroimaging abnormalities in the amygdala in mood disorders. Ann. N. Y. Acad. Sci. 985, 420–444.
Manji H. K. and Duman R. S. (2001) Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics. Psychopharmacol. Bull. 35, 5–49.
Drevets W. C. (2001) Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr. Opin. Neurobiol. 11, 240–249.
Strakowski S. M., Adler C. M., and DelBello M. P. (2002) tric MRI studies of mood disorders: do they distinguish unipolar and bipolar disorder? Bipolar Disord. 4, 80–88.
Beyer J. L. and Krishnan K. R. (2002) tric brain imaging findings in mood disorders. Bipolar Disorders 4, 89–104.
Cotter D., Mackay D., Landau S., Kerwin R., and Everall I. (2001) Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch. Gen. Psychiatry 58, 545–553.
Rajkowska G. (2002) Cell pathology in bipolar disorder. Bipolar Disord 4, 129–116.
Soares J. C. and Mann J. J. (1997) The anatomy of mood disorders—review of structural neuroimaging studies. Biol. Psychiatry 41, 86–106.
Stoll A. L., Renshaw P. F., Yurgelun-Todd D. A., and Cohen B. M. (2000) Neuroimaging in bipolar disorder: what have we learned? Biol. Psychiatry 48, 505–517.
Kessler R. C. (1997) The effects of stressful life events on depression. Annu. Rev. Psychol. 48, 191–214.
McEwen B. S. (1999) Stress and hippocampal plasticity. Annu. Rev. Neurosci. 22, 105–122.
Lee A. L., Ogle W. O., and Sapolsky R. M. (2002) Stress and depression: possible links to neuron death in the hippocampus. Bipolar Disorders 4, 117–128.
Sapolsky R. M. (2001) Depression, antidepressants, and the shrinking hippocampus. Proc. Natl. Acad. Sci. USA 98, 12,320–12,322.
Sapolsky R. M. (2000) Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch. Gen. Psychiatry 57, 925–935.
Malberg J. E. and Duman R. S. (2003) Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology 28, 1562–1571.
Pham K., Nacher J., Hof P. R., and McEwen B. S. (2003) Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur. J. Neurosci. 17, 879–886.
Gould E., Tanapat P., Rydel T., and Hastings N. (2000) Regulation of hippocampal neurogenesis in adulthood. Biol. Psychiatry 48, 715–720.
Bhat R. V., Shanley J., Correll M. P., et al. (2000) Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration. Proc. Natl. Acad. Sci. USA 97, 11,074–11,079.
Cimarosti H., Rodnight R., Tavares A., et al. (2001) An investigation of the neuroprotective effect of lithium in organotypic slice cultures of rat hippocampus exposed to oxygen and glucose deprivation. Neurosci. Lett. 315, 33–36.
D’Mello S. R., Anelli R., and Calissano P. (1994) Lithium induces apoptosis in immature cerebellar granule cells but promotes survival of mature neurons. Exp. Cell. Res. 211, 332–338.
Khodorov B., Pinelis V., Vinskaya N., Sorokina E., Grigortsevich N., and Storozhevykh T. (1999) Li+ protects nerve cells against destabilization of Ca2+ homeostasis and delayed death caused by removal of external Na+. FEBS Lett. 448, 173–176.
Nonaka S., Hough C. J., and Chuang D. M. (1998) Chronic lithium treatment robustyl protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-D-aspartate receptor-mediated calcium influx. Proc. Natl. Acad. Sci. USA 95, 2642–2647.
Hashimoto R., Hough C., Nakazawa T., Yamamoto T., and Chuang D. M. (2002) Lithium protection against glutamate excitotoxicity in rat cerebral cortical neurons: involvement of NMDA receptor inhibition possibly by decreasing NR2B tyrosine phosphorylation. J. Neurochem. 80, 589–597.
Kanai H., Chalecka-Franaszek E., Chen R. W., Hashimoto R., Hiroi T., and Chuang D. M. (2001) Valproic acid protects against glutamate-induced excitotoxicity in mature cerebellar granule cells. Society for Neuroscience Annual Meeting Abstract 94.18.
Chen R. W. and Chuang D. M. (1999) Long term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expression. A prominent role in neuroprotection against excitotoxicity. J. Biol. Chem. 274, 6039–6042.
Centeno F., Mora A., Fuentes J. M., Soler G., and Claro E. (1998) Partial lithium-associated protection against apoptosis induced by C2-ceramide in cerebellar granule neurons. Neuroreport 9, 4199–4203.
Nonaka S., Katsube N., and Chuang D. M. (1998) Lithium protects rat cerebellar granule cells against apoptosis induced by anticonvulsants, phenytoin and carbamazepine. J. Pharmacol. Exp. Ther. 286, 539–547.
Jeong M. R., Hashimoto R., Senatorov V. V., et al. (2003) Valproic acid, a mood stabilizer and anticonvulsant, protects rat cerebral cortical neurons from spontaneous cell death: a role of histone deacetylase inhibition. FEBS Lett. 542, 74–78.
Alvarez G., Munoz-Montano J. R., Satrustegui J., Avila J., Bogonez E., and Diaz-Nido J. (1999) Lithium protects cultured neurons against beta-amyloid-induced neurodegeneration. FEBS Lett. 453, 260–264.
Chuang D. M., Chen R., Chalecka-Franaszek E., et al. (2002) Neuroprotective effects of lithium in cultured cells and animal models of diseases. Bipolar Disorders 4, 129–136.
Chen G., Zeng W. Z., Yuan P. X., et al. (1999) The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J. Neurochem. 72, 879–882.
Nonaka S. and Chuang D. M. (1998) Neuroprotective effects of chronic lithium on focal cerebral ischemia in rats. Neuroreport 9, 2081–2084.
Ren M., Senatorov V. V., Chen R. W., and Chuang D. M. (2003) Postinsult treatment with lithium reduces brain damage and facilitates neurological recovery in a rat ischemia/reperfusion model. Proc. Natl. Acad. Sci. USA 100, 6210–6215.
Ren M., Leng Y., Jeong M. R., Leeds P., and Chuang D. M. (2004) Valproaic acid reduces brain damage induced by transient focal cerebral ischemia in rats: potential roles of histone deacetylase inhibition and heat shock protein induction. J. Neurochem. 89, 1358–1367.
Wei H., Qin Z. H., Senatorov V. V., et al. (2001) Lithium suppresses excitotoxicity-induced striatal lesions in a rat model of Huntington’s disease. Neuroscience 106, 603–612.
Cameron H. A., Hazel T. G., and McKay R. D. (1998) Regulation of neurogenesis by growth factors and neurotransmitters. J. Neurobiol. 36, 287–306.
Gage F. H. (2000) Mammalian neural stem cells. Science 287, 1433–1438.
Jacobs B. L. (2002) Adult brain neurogenesis and depression. Brain. Behav. Immun. 16, 602–609.
Jacobs B. L., Praag H., and Gage F. H. (2000) Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol. Psychiatry 5, 262–269.
Magavi S. S. and Macklis J. D. (2001) Manipulation of neural precursors in situ: induction of neurogenesis in the neocortex of adult mice. Neuropsychopharmacology 25, 816–835.
Duman R. S., Nakagawa S., and Malberg J. (2001) Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology 25, 836–844.
Malberg J. E., Eisch A. J., Nestler E. J., and Duman R. S. (2000) Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 20, 9104–9110.
Manev H., Uz T., Smalheiser N. R., and Manev R. (2001) Antidepressants alter cell proliferation in the adult brain in vivo and in neural cultures in vitro. Eur. J. Pharmacol. 411, 67–70.
Santarelli L., Saxe M., Gross C., et al. (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809.
Hashimoto R., Senatorov V., Kanai H., Leeds P., and Chuang D. M. (2003) Lithium stimulates progenitor proliferation in cultured brain neurons. Neuroscience 117, 55–61.
Chen G., Rajkowska G., Du F., Seraji-Bozorgzad N., and Manji H. K. (2000) Enhancement of hippocampal neurogenesis by lithium. J. Neurochem. 75, 1729–1734.
Hao Y., Creson T., Zhang L., et al. (2004) Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J. Neurosci. 24, 6590–6599.
McAllister A. K. (2002) Neurotrophins and cortical development. Results Probl. Cell. Differ. 39, 89–112.
McAllister A. K., Katz L. C., and Lo D. C. (1999) Neurotrophins and synaptic plasticity. Annu. Rev. Neurosci. 22, 295–318.
McAllister A. K. (2001) Neurotrophins and neuronal differentiation in the central nervous system. Cell. Mol. Life Sci. 58, 1054–1060.
Rasmusson A. M., Shi L., and Duman R. (2002) Downregulation of BDNF mRNA in the hippocampal dentate gyrus after re-exposure to cues previously associated with footshock. Neuropsychopharmacology 27, 133–142.
Smith M. A. and Cizza G. (1996) Stress-induced changes in brain-derived neurotrophic factor expression are attenuated in aged Fischer 344/N rats. Neurobiol. Aging 17, 859–864.
Ueyama T., Kawai Y., Nemoto K., Sekimoto M., Tone S., and Senba E. (1997) Immobilization stress reduced the expression of neurotrophins and their receptors in the rat brain. Neurosci. Res. 28, 103–110.
Smith M. A., Makino S., Kvetnansky R., and Post R. M. (1995) Effects of stress on neurotrophic factor expression in the rat brain. Ann. NY Acad. Sci. 771, 234–239.
Nibuya M., Morinobu S., and Duman R. S. (1995) Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 15, 7539–7547.
Chen B., Dowlatshahi D., MacQueen G. M., Wang J. F., and Young L. T. (2001) Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol. Psychiatry 50, 260–265.
Shirayama Y., Chen A. C., Nakagawa S., Russell D. S., and Duman R. S. (2002) Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 22, 3251–3261.
Fukumoto T., Morinobu S., Okamoto Y., Kagaya A., and Yamawaki S. (2001) Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain. Psychopharmacology (Berl) 158, 100–106.
Hashimoto R., Takei N., Shimazu K., Christ L., Lu B., and Chuang D. M. (2002) Lithium induces brain-derived neurotrophic factor and activates TrkB in rodent cortical neurons: an essential step for neuroprotection against glutamate excitotoxicity. Neuropharmacology 43, 1173–1179.
Yu I. T., Kim J. S., Lee S. H., Lee Y. S., and Son H. (2003) Chronic lithium enhances hippocampal long-term potentiation, but not neurogenesis, in the aged rat dentate gyrus. Biochem. Biophys. Res. Commun. 303, 1193–1198.
Son H., Yu I. T., Hwang S. J., et al. (2003) Lithium enhances long-term potentiation independently of hippocampal neurogenesis in the rat dentate gyrus. J. Neurochem. 85, 872–881.
Schlessinger J. (2000) Cell signaling by receptor tyrosine kinases. Cell 103, 211–225.
Poo M. M. (2001) Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2, 24–32.
Patapoutian A. and Reichardt L. F. (2001) Trk receptors: mediators of neurotrophin action. Curr. Opin. Neurobiol. 11, 272–280.
Yuan P. X., Huang L. D., Jiang Y. M., Gutkind J. S., Manji H. K., and Chen G. (2001) The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J. Biol. Chem. 276, 31,674–31,683.
Manji H. K., Moore G. J., Rajkowska G., and Chen G. (2000) Neuroplasticity and cellular resilience in mood disorders. Mol. Psychiatry 5, 578–593.
Huang X., Wu D. Y., Chen G., Manji H., and Chen D. F. (2003) Support of retinal ganglion cell survival and axon regeneration by lithium through a Bcl-2-dependent mechanism. Invest. Ophthalmol. Vis. Sci. 44, 347–354.
Quiroz J., Singh J., Gould T. D., Denicoff K. D., Zarate C. A., and Manji H. K. (2004) Emerging experimental therapeutics for bipolar disorder: clues from the molecular pathophysiology. Mol. Psychiatry. 9, 734–755.
De Sarno P., Li X., and Jope R. S. (2002) Regulation of Akt and glycogen synthase kinase-3beta phosphorylation by sodium valproate and lithium. Neuropharmacology 43, 1158–1164.
Kato T. and Kato N. (2000) Mitochondrial dysfunction in bipolar disorder. Bipolar Disord. 2, 180–190.
Tseng W. P. and Lin-Shiau S. Y. (2002) Long-term lithium treatment prevents neurotoxic effects of beta-bungarotoxin in primary cultured neurons. J. Neurosci. Res. 69, 633–641.
Wang J. F., Azzam J. E., and Young L. T. (2003) Valproate inhibits oxidative damage to lipid and protein in primary cultured rat cerebrocortical cells. Neuroscience 116, 485–489.
Pivovarova N. B., Pozzo-Miller L. D., Hongpaisan J., and Andrews S. B. (2002) Correlated calcium uptake and release by mitochondria and endoplasmic reticulum of CA3 hippocampal dendrites after afferent synaptic stimulation. J. Neurosci. 22, 10,653–10,661.
Williams J. M., Thompson V. L., Mason-Parker S. E., Abraham W. C., and Tate W. P. (1998) Synaptic activity-dependent modulation of mitochondrial gene expression in the rat hippocampus. Brain. Res. Mol. Brain Res. 60, 50–56.
Mattson M. P. and Liu D. (2003) Mitochondrial potassium channels and uncoupling proteins in synaptic plasticity and neuronal cell death. Biochem. Biophys. Res. Commun. 304, 539–549.
Murphy A. N., Bredesen D. E., Cortopassi G., Wang E., and Fiskum G. (1996) Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Proc. Natl. Acad. Sci. USA 93, 9893–9898.
Duchen M. R. (2000) Mitochondria and Ca(2+)in cell physiology and pathophysiology. Cell Calcium 28, 339–348.
Hoshi M., Sato M., Kondo S., et al. (1995) Different localization of tau protein kinase I/glycogen synthase kinase-3 beta from glycogen synthase kinase-3 alpha in cerebellum mitochondria. J. Biochem. (Tokyo) 118, 683–685.
King T. D., Bijur G. N., and Jope R. S. (2001) Caspase-3 activation induced by inhibition of mitochondrial complex I is facilitated by glycogen synthase kinase-3beta and attenuated by lithium. Brain. Res. 919, 106–114.
Dumont P., Leu J. I., Della Pietra A. C. 3rd, George D. L., and Murphy M. (2003) The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat. Genet. 33, 357–365.
Marchenko N. D., Zaika A., and Moll U. M. (2000) Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J. Biol. Chem. 275, 16,202–16,212.
Sansome C., Zaika A., Marchenko N. D., and Moll U. M. (2001) Hypoxia death stimulus induces translocation of p53 protein to mitochondria. Detection by immunofluorescence on whole cells. FEBS Lett. 488, 110–115.
Watcharasit P., Bijur G. N., Song L., Zhu J., Chen X., and Jope R. S. (2003) Glycogen synthase kinase-3beta (GSK3beta) binds to and promotes the actions of p53. J. Biol. Chem. 278, 48,872–48,879.
Bijur G. N. and Jope R. S. (2003) Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J. Neurochem. 87, 1427–1435.
Linseman D. A., Butts B. D., Precht T. A., et al. (2004) Glycogen synthase kinase-3beta phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J. Neurosci. 24, 9993–10,002.
Moore G. J., Bebchuk J. M., Hasanat K., et al. (2000) Lithium increases N-acetyl-aspartate in the human brain: in vivo evidence in support of bcl-2’s neurotrophic effects? Biol. Psychiatry 48, 1–8.
Moore G. J., Bebchuk J. M., Wilds I. B., Chen G., and Manji H. K. (2000) Lithium-induced increase in human brain grey matter. Lancet 356, 1241,1242.
Silverstone P. H., Wu R. H., O’Donnell T., Ulrich M., Asghar S. J., and Hanstock C. C. (2003) Chronic treatment with lithium, but not sodium valproate, increases cortical N-acetylaspartate concentrations in euthymic bipolar patients. Int. Clin. Psychopharmacol. 18, 73–79.
Sassi R., Nicoletti M., Brambilla P., et al. (2002) Increased gray matter in lithium-treated bipolar disorder patients. Neurosci. Lett. 329, 243.
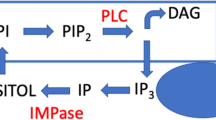
Atack J. R. (1997) Inositol monophosphatase inhibitors—lithium mimetics? Med. Res. Rev. 17, 215–224.
Atack J. R., Cook S. M., Watt A. P., Fletcher S. R., and Ragan C. I. (1993) In vitro and in vivo inhibition of inositol monophosphatase by the bisphosphonate L-690,330. J. Neurochem. 60, 652–658.
Atack J. R., Prior A. M., Fletcher S. R., Quirk K., McKernan R., and Ragan C. I. (1994) Effects of L-690,488, a prodrug of the bisphosphonate inositol monophosphatase inhibitor L-690,330, on phosphatidylinositol cycle markers. J. Pharmacol. Exp. Ther. 270, 70–76.
Pollack S. J., Atack J. R., Knowles M. R., et al. (1994) Mechanism of inositol monophosphatase, the putative target of lithium therapy. Proc. Natl. Acad. Sci. USA 91, 5766–5770.
Bone R., Springer J. P., and Atack J. R. (1992) Structure of inositol monophosphatase, the putative target of lithium therapy. Proc. Natl. Acad. Sci. USA 89, 10,031–10,035.
Chen S. J., Sweatt J. D., and Klann E. (1997) Enhanced phosphorylation of the postsynaptic protein kinase C substrate RC3/neurogranin during long-term potentiation. Brain. Res. 749, 181–187.
Conn P. J. and Sweatt J. D. (1994) Protein kinase C in the nervous system. In: Protein Kinase C, Kuo J. F., ed. New York: Oxford University Press, pp. 199–235.
Manji H. K. and Chen G. (2002) PKC, MAP kinases and the bcl-2 family of proteins as long-term targets for mood stabilizers. Mol. Psychiatry 7 (Suppl 1), S46-S56.
Bebchuk J. M., Arfken C. L., Dolan-Manji S., Murphy J., Hasanat K., and Manji H. K. (2000) A preliminary investigation of a protein kinase C inhibitor in the treatment of acute mania. Arch. Gen. Psychiatry 57, 95–97.
Horgan K., Cooke E., Hallett M. B., and Mansel R. E. (1986) Inhibition of protein kinase C mediated signal transduction by tamoxifen. Importance for antitumour activity. Biochem. Pharmacol. 35, 4463–4465.
O’Brian C. A., Housey G. M., and Weinstein I. B. (1988) Specific and direct binding of protein kinase C to an immobilized tamoxifen analogue. Cancer Res. 48, 3626–3629.
Frank R. N. (2002) Potential new medical therapies for diabetic retinopathy: protein kinase C inhibitors. Am. J. Ophthalmol. 133, 693–698.
Wheeler G. D. (2003) Ruboxistaurin (Eli Lilly). IDrugs 6, 159–163.
Aiello L. P. (2002) The potential role of PKC beta in diabetic retinopathy and macular edema. Surv. Ophthalmol. 47 (Suppl 2), S263-S269.
Parker P. J. (1999) Inhibition of protein kinase C—do we, can we, and should we? Pharmacol. Ther. 82, 263–267.
Kaidanovich O. and Eldar-Finkelman H. (2002) The role of glycogen synthase kinase-3 in insulin resistance and Type 2 diabetes. Expert Opin. Ther. Targets 6, 555–561.
Bhat R. V. and Budd S. L. (2002) GSK3beta signalling: casting a wide net in Alzheimer’s disease. Neurosignals 11, 251–261.
Alvarez G., Munoz-Montano J. R., Satrustegui J., Avila J., Bogonez E., and Diaz-Nido J. (2002) Regulation of tau phosphorylation and protection against beta-amyloid-induced neurodegeneration by lithium. Possible implications for Alzheimer’s disease. Bipolar Disord. 4, 153–165.
Sun X., Sato S., Murayama O., et al. (2002) Lithium inhibits amyloid secretion in COS7 cells transfected with amyloid precursor protein C100. Neurosci. Lett. 321, 61–64.
Tong H., Imahashi K., Steenbergen C., and Murphy E. (2002) Phosphorylation of glycogen synthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase—dependent pathway is cardioprotective. Circ. Res. 90, 377–379.
Frame S. and Cohen P. (2001) GSK3 takes centre stage more than 20 years after its discovery. Biochem. J. 359, 1–16.
Sasaki C., Hayashi T., Zhang W. R., et al. (2001) Different expression of glycogen synthase kinase-3beta between young and old rat brains after transient middle cerebral artery occlusion. Neurol. Res. 23, 588–592.
Dorronsoro I., Castro A., and Martinez A. (2002) Inhibitors of glycogen synthase kinase-3: future therapy for unmet medical needs. Expert Opin. Ther. Patents 12, 1527–1536.
Martinez A., Alonso M., Castro A., Perez C., and Moreno F. J. (2002) First non-ATP competitive glycogen synthase kinase 3 beta (GSK-3beta) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer’s disease. J. Med. Chem. 45, 1292–1299.
Plotkin B., Kaidanovich O., Talior I., and Eldar-Finkelman H. (2003) Insulin mimetic action of synthetic phosphorylated Peptide inhibitors of glycogen synthase kinase-3. J. Pharmacol. Exp. Ther. 305, 974–980.
Martinez A., Castro A., Dorronsoro I., and Alonso M. (2002) Glycogen synthase kinase 3 (GSK-3) inhibitors as new promising drugs for diabetes, neurodegeneration, cancer, and inflammation. Med. Res. Rev. 22, 373–384.
Cohen P. and Frame S. (2001) The renaissance of GSK3. Nat. Rev. Mol. Cell Biol. 2, 769–776.
Ilouz R., Kaidanovich O., Gurwitz D., and Eldar-Finkelman H. (2002) Inhibition of glycogen synthase kinase-3beta by bivalent zinc ions: insight into the insulin-mimetic action of zinc. Biochem. Biophys. Res. Commun. 295, 102–106.
D’Sa C. and Duman R. (2002) Antidepressants and neuroplasticity. Bipolar Disorder 4, 183.
Manji H. K., Quiroz J. A., Sporn J., et al. (2003) Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol. Psychiatry 53, 707–742.
Zeller E., Stief H. J., Pflug B., and Sastre-y-Hernandez M. (1984) Results of a phase II study of the antidepressant effect of rolipram. Pharmacopsychiatry 17, 188–190.
Bobon D., Breulet M., Gerard-Vandenhove M. A., et al. (1988) Is phosphodiesterase inhibition a new mechanism of antidepressant action? A double blind double-dummy study between rolipram and desipramine in hospitalized major and/or endogenous depressives. Eur. Arch. Psychiatry. Neurol. Sci. 238, 2–6.
Hebenstreit G. F., Fellerer K., Fichte K., et al. (1989) Rolipram in major depressive disorder: results of a double-blind comparative study with imipramine. Pharmacopsychiatry 22, 156–160.
Zhu J., Mix E., and Winblad B. (2001) The antidepressant and antiinflammatory effects of rolipram in the central nervous system. CNS Drug. Rev. 7, 387–398.
Marks P., Rifkind R. A., Richon V. M., Breslow R., Miller T., and Kelly W. K. (2001) Histone deacetylases and cancer: causes and therapies. Nat. Rev. Cancer. 1, 194–202.
Ketter T. A. and Wang P. W. (2003) The emerging differential roles of GABAergic and antiglutamatergic agents in bipolar disorders. J. Clin. Psychiatry 64(Suppl 3), 15–20.
Plotsky P. M., Owens M. J., and Nemeroff C. B. (1998) Psychoneuroendocrinology of depression. Hypothalamic-pituitary-adrenal axis. Psychiatr. Clin. North. Am. 21, 293–307.
Gold P. W. and Chrousos G. P. (2002) Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol. Psychiatry 7, 254–275.
Seymour P. A., Schmidt A. W., and Schulz D. W. (2003) The pharmacology of CP-154,526, a non-peptide antagonist of the CRH1 receptor: a review. CNS Drug Rev. 9, 57–96.
Mansbach R. S., Brooks E. N., and Chen Y. L. (1997) Antidepressant-like effects of CP-154,526, a selective CRF1 receptor antagonist. Eur. J. Pharmacol. 323, 21–26.
Miner J. N., Tyree C., Hu J., et al. (2003) A non-steroidal glucocorticoid receptor antagonist. Mol. Endocrinol. 17, 117–127.
Honer C., Nam K., Fink C., et al. (2003) Glucocorticoid receptor antagonism by cyproterone acetate and RU486. Mol. Pharmacol. 63, 1012–1020.
Ur E., Turner T. H., Goodwin T. J., Grossman A., and Besser G. M. (1992) Mania in association with hydrocortisone replacement for Addison’s disease. Postgrad. Med. J. 68, 41–43.
Salek F. S., Bigos K. L., and Kroboth P. D. (2002) The influence of hormones and pharmaceutical agents on DHEA and DHEA-S concentrations: a review of clinical studies. J. Clin. Pharmacol. 42, 247–266.
Zarate C. A., Quiroz J., Payne J., and Manji H. K. (2002) Modulators of the glutamatergic system: implications for the development of improved therapeutics in mood disorders. Psychopharmacol. Bull. 36, 35–83.
Einat H., Manji H. K., and Belmater R. H. (2003) New approaches to modeling bipolar disorder. Psychopharm. Bull. 37, 47–63.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bachmann, R.F., Schloesser, R.J., Gould, T.D. et al. Mood stabilizers target cellular plasticity and resilience cascades. Mol Neurobiol 32, 173–202 (2005). https://doi.org/10.1385/MN:32:2:173
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/MN:32:2:173