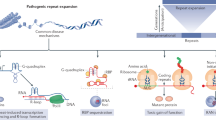
Abstract
Triplet repeat expansions were first discovered in 1991 and since then have been found to be the mutation underlying a range of neurodegenerative, neuromuscular, and cognitive disorders including fragile X syndrome, myotonic dystrophy, Friedreich's ataxia, and the polyglutamine disorders that include Huntington's disease. The repeats exert their detrimental effects through different molecular mechanisms dependent on whether they are located in coding or noncoding regions of the gene in question. During the past 10 yr, a wide range of strategies have been used to successfully establish mouse models for all of these disorders. This review presents an overview of these mouse models, discusses the insights into the molecular pathogenesis of these disorders that have been gained from their analysis and the strategies that are being used to uncover novel therapeutic options.
Similar content being viewed by others
References
Verker, A. J., Pieretti, M., Sutcliffe, J. S., et al. (1991) Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65, 905–914.
La Spada, A. R., Wilson, E. M., Lubahn, D. B., Harding, A. E., and Fischbeck, K. H. (1991) Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352, 77–79.
Campuzano, V., Montermini, L., Molto, M. D., et al. (1996) Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271, 1423–1427.
Gusella, J. F. and MacDonald, M. E. (2000) Molecular genetics: unmasking polyglutamine triggers in neurodegenerative disease. Nat. Rev. Neurosci. 1, 109–115.
Mankodi, A. and Thornton, C. A. (2002) Myotonic syndromes. Curr. Opin. Neurol. 15, 545–552.
Koob, M. D., Moseley, M. L., Schut, L. J., et al. (1999) An untranslated CTG expansion causes a novel form of spinocerebellar ataxia (SCA8). Nat. Genet. 21, 379–384.
O'Donnell, W. T. and Warren, S. T. (2002) A decade of molecular studies of fragile X syndrome. Annu. Rev. Neurosci. 25, 315–338.
Jin, P. and Warren, S. T. (2003) New insights into fragile X syndrome: from molecules to neurobehaviors. Trends Biochem. Sci. 28, 152–158.
The Dutch-Belgian Fragile X Consortium (1994) Fmrl knockout mice: a model to study fragile X mental retardation. Cell 78, 23–33.
Frank Kooy, R. (2003) Of mice and the fragile X syndrome. Trends Genet. 19, 148–154.
Frankland, P. W., Wang, Y., Rosner, B., et al. (2004) Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmrl-knockout mice. Mol. Psychiatry 9, 417–425.
Slegtenhorst-Eegdeman, K. E., de Rooij, D. G., Verhoef-Post, M., et al. (1998) Macroorchidism in FMR1 knockout mice is caused by increased Sertoli cell proliferation during testicular development. Endocrinology 139, 156–162.
Comery, T. A., Harris, J. B., Willems, P. J., et al. (1997) Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc. Natl. Acad. Sci. USA 94, 5401–5404.
Irwin, S. A., Idupulapati, M., Gilbert, M. E., et al. (2002) Dendritic spine and dendritic field characteristics of layer V pyramidal neurons in the visual cortex of fragile-X knockout mice. Am. J. Med. Genet. 111, 140–146.
Galvez, R., Gopal, A. R. and Greenough, W. T. (2003) Somatosensory cortical barrel dendritic abnormalities in a mouse model of the fragile X mental retardation syndrome. Brain Res. 971, 83–89.
Chen, L. and Toth, M. (2001) Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience 103, 1043–1050.
Hagerman, R. J., Leehey, M., Heinrichs, W., et al. (2001) Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology 57, 127–130.
Hagerman, P. J. and Hagerman, R. J. (2004) Fragile X-associated tremor/ataxia syndrome (FXTAS)? Ment. Retard. Dev. Disabil. Res. Rev. 10, 25–30.
Jacquemont, S., Hagerman, R. J., Leehey, M., et al. (2003) Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am. J. Hum. Genet. 72, 869–878.
Tassone, F., Hagerman, R. J., Taylor, A. K., Gane, L. W., Godfrey, T. E., and Hagerman, P. J. (2000) Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am. J. Hum. Genet. 66, 6–15.
Kenneson, A., Zhang, F., Hagedorn, C. H., and Warren, S. T. (2001) Reduced FMRP and increased FMR1 transciption is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum. Mol. Genet., 10, 1449–1454.
Greco, C. M., Hagerman, R. J., Tassone, F., et al. (2002) Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain 125, 1760–1771.
Bontekoe, C. J., Bakker, C. E., Nieuwenhiizen, I. M. et al. (2001) Instability of a (CGG)98 repeat in the Fmrl promoter. Hum. Mol. Genet. 10, 1693–1699.
Willemsen, R., Hoogeveen-Westerveld, M., Reis, S., et al. (2003) The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions: implications for the cerebellar tremor/ataxia syndrome. Hum. Mol. Genet. 12, 949–959.
Van Dam, D., Errijgers, V., Kooy, R. F., et al. (2005) Cognitive decline, neuromotor and behavioural disturbances in a mouse model for fragile-X-associated tremor/ataxia syndrome (FXTAS). Behav. Brain Res. 162, 233–239.
Patel, P. I. and Isaya, G. (2001) Freidreich ataxia: from GAA triplet-repeat expansion to frataxin deficiency. Am. J. Hum. Genet. 69, 15–24.
Puccio, H. and Koenig, M. (2002) Freidreich ataxia: a paradigm for mitochondrial diseases. Curr. Opin. Genet. Dev. 12, 272–277.
Cossee, M., Puccio, H., Gansmuller, A., et al. (2000) Inactivation of the Friedreich ataxia mouse gene leads to early embryonic lethality without iron accumulation. Hum. Mol. Genet. 9, 1219–1226.
Puccio, H., Simon, D., Cossee, M., et al. (2001) Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe-S enzyme deficiency followed by intramitochondrial iron deposits. Nat. Genet. 27, 181–186.
Simon, D., Seznec, H., Gansmuller, A., et al. (2004) Friedreich ataxia mouse models with progressive cerebellar and sensory ataxia reveal autophagic neurodegeneration in dorsal root ganglia. J. Neurosci. 24, 1987–1995.
Miranda, C. J., Santos, M. M., Ohshima, K., et al. (2002) Frataxin knockin mouse. FEBS Lett. 512, 291–297.
Pook, M. A., Al-Mahdawi, S., Carroll, C. J., et al. (2001) Rescue of the Freidreich's ataxia knockout mouse by human YAC transgenesis. Neurogenetics 3, 185–193.
Sarsero, J. P., Li, L., Holloway, T. P., et al. (2004) Human BAC-mediated rescue of the Friedreich ataxia knockout mutation in transgenic mice. Mamm. Genome 15, 370–382.
Miranda, C. J., Santos, M. M., Ohshima, K., Tessaro, M., Sequeiros, J., and Pandolfo, M. (2004) Frataxin overexpressing mice. FEBS Lett. 572, 281–288.
Meola, G. (2000) Myotonic dystrophies. Curr. Opin. Neurol. 13, 519–525.
Ranum, L. P. and Day, J. W. (2002) Dominantly inherited, non-coding microsatellite expansion disorders. Curr. Opin. Genet. Dev. 12, 266–271.
Reddy, S., Smith, D. B., Rich, M. M., et al. (1996) Mice lacking the myotonic dystrophy protein kinase develop a late onset progressive myopathy. Nat. Genet. 13, 325–335.
Jansen, G., Groenen, P. J., Bachner, D., et al. (1996) Abnormal myotonic dystrophy protein kinase levels produce only mild myopathy in mice. Nat. Genet. 13, 316–324.
Mounsey, J. P., Mistry, D. J., Ai, C. W., Reddy, S. and Moorman, J. R. (2000) Skeletal muscle sodium channel gating in mice deficient in myotonic dystrophy protein kinase. Hum. Mol. Genet. 9, 2313–2320.
Berul, C. I., Maguire, C. T., Aronovitz, M. J., et al. (1999) DMPK dosage alterations result in atrioventricular conduction abnormalities in a mouse myotonic dystrophy model. J. Clin. Invest. 103, R1-R7.
Sarkar, P. S., Appukuttan, B., Han, J., et al. (2000) Heterozygous loss of Six5 in mice is sufficient to cause ocular cataracts, Nat. Genet. 25, 110–114.
Klesert, T. R., Cho, D. H., Clark, J. I., et al. (2000) Mice deficient in Six5 develop cataracts: implications for myotonic dystrophy. Nat. Genet. 25, 105–109.
Wakimoto, H. Maguire, C. T., Sherwood, M. C., et al. (2002) Characterization of cardiac conduction system abnormalities in mice with targeted disruption of Six5 gene. J. Interv. Card. Electrophysiol. 7, 127–135.
Personius, K. E., Nautiyal, J., and Reddy, S. (2005) Myotonia and muscle contractile properties in mice with SIX5 deficiency. Muscle Nerve 31, 503–505.
Liquori, C. L., Ricker, K., Moseley, M. L., et al. (2001) Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science 293, 864–867.
Ranum, L. P. and Day, J. W. (2004) Pathogenic RNA repeats: an expanding role in genetic disease. Trends Genet. 20, 506–512.
Mankodi, A., Logigian, E., Callahan, L., et al., (2000) Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science 289, 1769–1773.
Mankodi, A., Takahashi, M. P., Jiang, H., et al. (2002) Expanded CUG repeats trigger aberrant splicing of CIC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol. Cell 10, 35–44.
Seznec, H., Agbulut, O., Sergeant, N., et al. (2001) Mice transgenic for the human myotonic dystrophy region with expanded CTG repeats display muscular and brain abnormalities. Hum. Mol. Genet. 10, 2717–2726.
Storbeck, C. J., Drmanic, S., Daniel, K., et al. (2004) Inhibition of myogenesis in transgenic mice expressing the human DMPK 3′-UTR. Hum. Mol. Genet. 13, 589–600.
Kanadia, R. N., Johnstone, K. A., Mankodi, A., et al. (2003) A muscleblind knockout model for myotonic dystrophy. Science 302, 1978–1980.
Ho, T. H., Bundman, D., Armstrong, D. L., and Cooper, T. A. (2005) Transgenic mice expressing CUG-BP1 reproduce s;licing mis-regulation observed in myotonic dystrophy. Hum. Mol. Genet. 14, 1539–1547.
Mangiarini, L., Sathasivam, K., Seller, M., et al. (1996) Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87, 493–506.
Schilling, G., Becher, M. W., Sharp, A. H., et al. (1999) Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin [published erratum appears in Hum. Mol. Genet.. 1999;8:943]. Hum. Mol. Genet. 8, 397–407.
Laforet, G. A., Sapp, E., Chase, K., et al. (2001) Changes in cortical and striatal neurons predict behavioral and electrophysiological abnormalities in a transgenic murine model of Huntington's disease. J. Neurosci. 21, 9112–9123.
Gu, X., Li, C., Wei, W., et al. (2005) Pathological cell-cell interactions elicited by a neuropathogenic form of mutant Huntingtin contribute to cortical pathogenesis in HD mice. Neuron 46, 433–444.
Reddy, P. H., Williams, M., Charles, V., et al. (1998) Behavioural abnormalities and selective neuronal loss in HD transgenic mice expressing mutated full-length HD cDNA. Nat. Genet. 20, 198–202.
Hodgson, J. G., Agopyan, N., Gutekunst, C. A., et al. (1999) A YAC mouse model for Huntington's disease with full-length mutant huntingtin, cytoplasmic toxicity, and selective striatal neurodegeneration. Neuron 23, 181–192.
Slow, E. J., van Raamsdonk, J., Rogers, D., et al. (2003) Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Hum. Mol. Genet. 12, 1555–1567.
Shelbourne, P. F., Killeen, N., Hevner, R. F., et al. (1999) A Huntington's disease CAG expansion at the murine Hdh locus is unstable and associated with behavioural abnormalities in mice. Hum. Mol. Genet. 8, 763–774.
Levine, M. S., Klapstein, G. J., Koppel, A., et al. (1999) Enhanced sensitivity to N-methyl-D-aspartate receptor activation in transgenic and knock in mouse models of Huntington's disease. J. Neurosci,. Res. 58, 515–532.
Wheeler, V. C., White, J. K., Gutekunst, C. A., et al. (2000) Long glutamine tracts cause nuclear localization of a novel form of huntingtin in medium spiny striatal neurons in H dhQ92 and HdhQ111 knock- in mice. Hum. Mol. Genet. 9., 503–513.
Lin, C. H., Tallaksen-Greene, S., Chien, W. M. et al. (2001) Neurological abnormalities in a knockin mouse model of Huntington's disease. Hum. Mol. Genet. 10, 137–144.
Burright, E. N., Clark, H. B., Servadio, A., et al. (1995) SCA1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell 82, 937–948.
Huynh, D. P., Figueroa, K., Hoang, N., and Pulst, S. M. (2000) Nuclear localization or inclusion body formation of ataxin-2 are not necessary for SCA2 pathogenesis in mouse or human. Nat. Genet. 26, 44–50.
Cemal, C. K., Carroll, C. J., Lawrence, L., et al. (2002) YAC transgenic mice carrying pathological alleles of the MJD1 locus exhibit a mild and slowly progressive cerebellar deficit. Hum. Mol. Genet. 11, 1075–1094.
Yvert, G., Lindenberg, K. S., Picaud, S., Landwehrmeyer, G. B., Sahel, J. A., and Mandel, J. L. (2000) Expanded polyglutamines induce neurode-generation and trans-neuronal alterations in cerebellum and retina of SCA7 transgenic mice. Hum. Mol. Genet. 9, 2491–2506.
Yvert, G., Lindenberg, K. S., Devys, D., Helmlinger, D., Landwehrmeyer, G. B., and Mandel, J. L. (2001) SCA7 mouse models show selective stabilization of mutant ataxin-7 and similar cellular responses in different neuronal cell types. Hum. Mol. Genet. 10, 1679–1692.
La Spada, A. R., Fu, Y., Sopher, B. L., et al. (2001) Polyglutamine-expanded ataxin-7 antagonizes crx function and induces cone-rod dystrophy in a mouse model of sca7. Neuron 31, 913–927.
Watase, K., Weeber, E. J., Xu, B., et al. (2002) A long CAG repeat in the mouse Sca1 locus replicates SCA1 features and reveals the impact of protein solubility on selective neurodegeneration. Neuron 34, 905–919.
Yoo, S. Y., Pennesi, M. E., Weeber, E. J., et al. (2003) SCA7 knockin mice model human SCA7 and reveal gradual accumulation of mutant ataxin-7 in neurons and abnormalities in short-term plasticity. Neuron, 37, 383–401.
Schilling, G., Wood, J. D., Duan, K., et al. (1999) Nuclear accumulation of truncated atrophin-1 fragments in a transgenic mouse model of DRPLA. Neuron 24, 275–286.
Sato, T., Oyake, M., Nakamura, K., et al. (1999) Transgenic mice harboring a full-length human mutant DRPLA gene exhibit age-dependent intergenerational and somatic instabilities of CAG repeats comparable with those in DRPLA patients. Hum. Mol. Genet. 8, 99–106.
Abel, A., Walcott, J., Woods, J., Duda, J., and Merry, D. E. (2001) Expression of expanded repeat androgen receptor produces neurologic disease in transgenic mice. Hum. Mol. Genet. 10, 107–116.
Adachi, H., Kume, A., Li, M., et al. (2001) Transgenic mice with an expanded CAG repeat controlled by the human AR promoter show polyglutamine nuclear inclusions and neuronal dysfunction without neuronal cell death. Hum. Mol. Genet. 10, 1039–1048.
Katsuno, M., Adachi, H., Kume, A., et al. (2002) Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron 35, 843–854.
McManamny, P., Chy, H. S., Finkelstein, D. I., et al. (2002) A mouse model of spinal and bulbar muscular atrophy. Hum. Mol. Genet. 11, 2103–2111.
Sopher, B. L., Thomas, P. S., Jr., LaFevre-Bernt, M. A., et al. (2004) Androgen receptor YAC transgenic mice recapitulate SBMA motor neuronopathy and implicate VEGF164 in the motor neuron degeneration, Neuron 41, 687–699.
Davies, S. W., Turmaine, M., Cozens, B. A., et al. (1997) Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 90, 537–548.
Li, H., Li, S. H., Cheng, A. L., Mangiarini, L., Bates, G. P., and Li, X. J. (1999) Ultrastructural localization and progressive formation of neuropil aggregates in Huntington's disease transgenic mice. Hum. Mol. Genet. 8, 1227–1236.
Skinner, P. J., Koshy, B. T., Cummings, C. J., et al. (1997) Ataxin-1 with an expanded glutamine tract alters nuclear matrix- associated structures [published erratum appears in Nature 1998;391:307]. Nature 389, 971–974.
DiFiglia, M., Sapp, E., Chase, K. O., et al. (1997) Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277, 1990–1993.
Gutekunst, C. A., Li, S. H., Yi, H., et al. (1999) Nuclear and neuropil aggregates in Huntington's disease: relationship to neuropathology. J. Neurosci. 19, 2522–2534.
Schilling, G., Jinnah, H. A., Gonzales, V., et al. (2001) Distinct behavioral and neuropathological abnormalities in transgenic mouse models of HD and DRPLA. Neurobiol. Dis. 8, 405–418.
Cummings, C. J., Sun, Y., Opal, P., et al. (2001) Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum. Mol. Genet. 10, 1511–1518.
Adachi, H., Katsuno, M., Minamiyama, M., et al. (2003) Heat shock protein 70 chaperone overexpression ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model by reducing nuclear-localized mutant androgen receptor protein. J. Neurosci. 23, 2203–2211.
Hansson, O., Nylandsted, J., Castilho, R. F., Leist, M., Jaattela, M., and Brundin, P. (2003) Overexpression of heat shock protein 70 in R6/2 Huntington's disease mice has only modest effects on disease progression. Brain Res. 970, 47–57.
Hay, D. G., Sathasivam, K., Tobaben, S., et al. (2004) Progressive decrease in chaperone protein levels in a mouse model of huntington's disease and induction of stress proteins as a therapeutic approach. Hum. Mol. Genet. 13, 1389–1405.
Helmlinger, D., Bonnet, J., Mandel, J. L., Trottier, Y., and Devys, D. (2004) Hsp70 and Hsp40 chaperones do not modulate retinal phenotype in SCA7 mice. J. Biol. Chem. 279, 55969–55977.
Cha, J. H., Kosinski, C. M., Kerner, J. A., et al. (1998) Altered brain neurotransmitter receptors in transgenic mice expressing a portion of an abnormal human huntington disease gene. Proc. Natl. Acad. Sci. USA 95, 6480–6485.
Luthi-Carter, R., Strand, A., Peters, N. L., et al. (2000) Decreased expression of striatal signaling genes in a mouse model of Huntington's disease. Hum. Mol. Genet. 9, 1259–1271.
Lin, X., Antalffy, B., Kang, D., Orr, H. T., and Zoghbi, H. Y. (2000) Polyglutamine expansion down-regulates specific neuronal genes before pathologic changes in SCA1. Nat. Neurosci. 3, 157–163.
Ferrante, R. J., Andreassen, O. A., Jenkins, B. G., et al. (2000) Neuroprotective effects of creatine in a transgenic mouse model of Huntington's disease. J. Neurosci. 20, 4389–4397.
Andreassen, O. A., Dedeoglu, A., Ferrante, R. J., et al. (2001) Creatine increase survival and delays motor symptoms in a transgenic animal model of Huntington's disease. Neurobiol. Dis. 8, 479–491.
Ferrante, R. J., Andreassen, O. A., Dedeoglu, A., et al. (2002) Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington's disease. J. Neurosci. 22, 1592–1599.
Schilling, G., Coonfield, M. L., Ross, C. A., and Borchelt, D. R. (2001) Coenzyme Q10 and remacemide hydrochloride ameliorate motor deficits in a Huntington's disease transgenic mouse model. Neurosci. Lett. 315, 149–153.
Schiefer, J., Landwehrmeyer, G. B., Luesse, H. G., et al. (2002) Riluzole prolongs survival time and alters nuclear inclusion formation in a transgenic mouse model of Huntington's disease. Mov. Disord. 17, 748–757.
Andreassen, O. A., Ferrante, R. J., Dedeoglu, A., and Beal, M. F. (2001) Lipoic acid improves survival in transgenic mouse models of Huntington's disease. Neuroreport 12, 3371–3373.
Klivenyi, P., Ferrante, R. J., Gardian, G., Browne, S., Chabrier, P. E., and Beal, M. F. (2003) Increased survival and neuroprotective effects of BN82451 in a transgenic mouse model of Huntington's disease. J. Neurochem. 86, 267–272.
Clifford, J. J., Drago, J., Natoli, A. L. et al. (2002) Essential fatty acids given from conception prevent topographies of motor deficit in a transgenic model of Huntington's disease. Neuroscience 109, 81–88.
Karpuj, M. V., Becher, M. W., Springer, J. E., et al. (2002) Prolonged survival and decreased abnormal movements in transgenic model of Huntington disease, with administration of the transglutaminase inhibitor cystamine. Nat. Med. 8, 143–149.
Dedeoglu, A., Kubilus, J. K., Jeitner, T. M., et al. (2002) Therapeutic effects of cystamine in a murine model of Huntington's disease. J. Neurosci. 22, 8942–8950.
Bailey, C. D. and Johnson, G. V. (2005) The protective effects of cystamine in the R6/2 Huntington's disease mouse involve mechanisms other than the inhibition of tissue transglutaminase. Neurobiol. Aging, in press.
Sanchez, I., Mahlke, C., and Yuan, J. (2003) Pivotal role of oligomerization in expanded polyglutamine neurodegenerative disorders. Nature 421, 373–379.
Tanaka, M., Machida, Y., Niu, S., et al. (2004) Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat. Med. 10, 148–154.
Hockly, E., Richon, V. M., Woodman, B., et al. (2003) Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington's disease. Proc. Natl. Acad. Sci. USA 100, 2041–2046.
Ferrante, R. J., Kubilus, J. K., Lee, J., et al. (2003) Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington's disease mice. J. Neurosci. 23, 9418–9427.
Gardian, G., Browne, S. E., Choi, D. K., et al. (2005) Neuroprotective effects of phenylbutyrate in the N171-82Q transgenic mouse model of Huntington's disease. J. Biol. Chem. 280, 556–563.
Ravikumar, B., Vacher, C., Berger, Z., et al. (2004) Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat. Genet. 36, 585–595.
Minamiyama, M., Katsuno, M., Adachi, H., et al. (2004) Sodium butyrate ameliorates phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Hum. Mol. Genet. 13, 1183–1192.
Chen, M., Ona, V. O., Li, M., et al. (2000) Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat. Med. 6, 797–801.
Smith, D. L., Woodman, B., Mahal, A., et al. (2003) Minocycline and doxycycline are not beneficial in a model of Huntington's disease. Ann. Neurol. 54, 186–196.
Heiser, V., Engemann, S., Brocker, W., et al., (2002) Identification of benzothiazoles as potential polyglutamine aggregation inhibitors of Huntington's disease by using an automated filter retardation assay. Proc. Natl. Acad. Sci. USA 99 Suppl 4, 16400–16406.
Katsuno, M., Adachi, H., Doyu, M., et al. (2003) Leuprorelin rescues polyglutamine-dependent phenotypes in a transgenic mouse model of spinal and bulbar muscular atrophy. Nat. Med. 9, 768–773.
Chevalier-Larsen, E. S., O'Brien, C. J., et al. (2004) Castration restores function and neurofilament alterations of aged symptomatic males in a transgenic mouse model of spinal and bulbar muscular atrophy. J. Neurosci. 24, 4778–4786.
Xia, H., Mao, Q., Eliason, S. L., et al. (2004) RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat. Med. 10, 816–820.
Harper, S. Q., Staber, P. D., He, X., et al. (2005) RNA interference improves motor and neuropathological abnormalities in a Huntington's disease mouse model. Proc. Natl. Acad. Sci. USA 102, 5820–5825.
Yamamoto, A., Lucas, J. J., and Hen, R. (2000) Reversal of neuropathology and motor dysfunction in a conditional model of Huntington's disease. Cell 101, 57–66.
Zu, T., Duvick, L. A., Kaytor, M. D., et al. (2004) Recovery from polyglutamine-induced neurodegeneration in conditional SCA1 transgenic mice. J. Neurosci. 24, 8853–8861.
Peier, A. M., and Nelson, D. L. (2002) Instability of a premutation-sized CGG repeat in FMR1 YAC transgenic mice. Genomics 80, 423–432.
Al-Mahdawi, S., Pinto, R. M., Ruddle, P., Carroll, C., Webster, Z., and Pook, M. (2004) GAA repeat instability in Friedreich ataxia YAC transgenic mice. Genomics 84, 301–310.
Mangiarini, L., Sathasivam, K., Mahal, A., Mott, R., Seller, M., and Bates, G. P. (1997) Instability of highly expanded CAG repeats in mice transgenic for the Huntington's disease mutation. Nat. Genet. 15, 197–200.
Wheeler, V. C., Auerbach, W., White, J. K., et al. (1999) Length-dependent gametic CAG repeat instability in the Huntington's disease knockin mouse. Hum. Mol. Genet. 8, 115–122.
Kaytor, M. D., Burright, E. N., Duvick, L. A., Zoghbi, H. Y., and Orr, H. T. (1997) Increased trinucleotide repeat instability with advanced maternal age. Hum. Mol. Genet. 6, 2135–2139.
Lorenzetti, D., Watase, K., Xu, B., Matzuk, M. M., Orr, H. T., and Zoghbi, H. Y. (2000) Repeat instability and motor incoordination in mice with a targeted expanded CAG repeat in the Sca1 locus. Hum. Mol. Genet. 9, 779–785.
Kennedy, L., and Shelbourne, P. F. (2000) Dramatic mutation instability in HD mouse striatum: does polyglutamine load contribute to cell-specific vulnerability in Huntington's disease? Hum. Mol. Genet. 9, 2539–2544.
Telenius, H., Kremer, B., Goldberg, Y. P., et al. (1994) Somatic and gonadal mosaicism of the Huntington disease gene CAG repeat in brain and sperm [published erratum appears in Nat. Genet. 1994;7:113]. Nat. Genet. 6, 409–414.
Kennedy, L., Evans, E., Chen, C. M., et al. (2003) Dramatic tissue-specific mutation length increases are an early molecular event in Huntington disease pathogenesis. Hum. Mol. Genet. 12, 3359–3367.
Monckton, D. G., Coolbaugh, M. I., Ashizawa, K. T., Siciliano, M. J., and Caskey, C. T. (1997) Hypermutable myotonic dystrophy CTG repeats in transgenic mice. Nat. Genet. 15, 193–196.
Seznec, H., Lia-Baldini, A. S., Duros, C., et al. (2000) Transgenic mice carrying large human genomic sequences with expanded CTG repeat mimic closely the DM CTG repeat intergenerational and somatic instability. Hum. Mol. Genet. 9, 1185–1194.
Fortune, M. T., Vassilopoulos, C., Coolbaugh, M. I., Siciliano, M. J., and Monckton, D. G. (2000) Dramatic, expansion-biased, age-dependent, tissue-specific somatic mosaicism in a transgenic mouse model of triplet repeat instability. Hum. Mol. Genet. 9, 439–445.
Pearson, C. E., Ewel, A., Acharya, S. Fishel, R. A., and Sinden, R. R. (1997) Human MSH2 binds to trinucleotide repeat DNA structures associated with neurodegenerative diseases. Hum. Mol. Genet. 6, 1117–1123.
Manley, K., Shirley, T. L., Flaherty, L., and Messer, A. (1999) Msh2 deficiency prevents in vivo somatic instability of the CAG repeat in Huntington disease transgenic mice. Nat. Genet. 23, 471–473.
Wheeler, V. C., Lebel, L. A., Vrbanac, V., Teed, A., Te Riele, H., and MacDonald, M. E. (2003) Mismatch repair gene Msh2 modifies the timing of early disease in Hdh(Q111) striatum. Hum. Mol. Genet. 12, 273–281.
van den Broek, W. J., Nelen, M. R., Wansink, D. G., et al. (2002) Somatic expansion behaviour of the (CTG)n repeat in myotonic dystrophy knockin mice is differentially affected by Msh3 and Msh6 mismatch-repair proteins. Hum. Mol. Genet. 11, 191–198.
Savouret, C., Brisson, E., Essers, J., et al. (2003) CTG repeat instability and size variation timing in DNA repair-deficient mice. EMBO J. 22, 2264–2273.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bates, G.P., Gonitel, R. Mouse models of triplet repeat diseases. Mol Biotechnol 32, 147–158 (2006). https://doi.org/10.1385/MB:32:2:147
Issue Date:
DOI: https://doi.org/10.1385/MB:32:2:147