Abstract
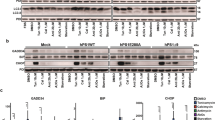
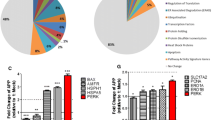
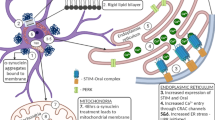
In vitro studies designed to probe the cellular mechanisms underlying β-amyloid (Aβ) toxicity in neurons have implicated several processes, including hyperphosphorylation of the microtubule (MT)-associated protein tau, loss of MT stability, and increased cytosolic calcium levels. Given that Alzheimer's disease involves accumulation of aggregates of two different proteins, the potential involvement of the unfolded protein response (UPR) and endoplasmic reticulum (ER) dysfunction has been suggested to lead to cell death. The relationship between these apparently divergent factors and pathways in Aβ toxicity is still unclear. In these studies we investigated the relationship between MT stability and the ER stress response in primary neurons exposed to toxic Aβ peptides in culture. In addition, nocodazole (ND) was used to determine if direct disruption of MT organization activated the UPR. Pretreatment of neurons with MT-stabilizing drugs paclitaxel (Taxol) and epothilone A prevented the induction of three indicators of the UPR induced by Aβ, ND, and thapsigargin, a compound known to inhibit the sarco-ER Ca2+-ATPase and deplete ER calcium stores, resulting in initiation of the UPR. In addition, treatment with MT-stabilizing drugs blocked cell death and the cytoskeletal disorganization induced by these insults. The results suggest that loss of cytoskeletal integrity is a very early step in the response to a variety of toxic stimuli and that preservation of MT stability might be important in preventing the induction of ER dysfunction and subsequent cell death by Aβ in neurons.
Similar content being viewed by others
References
Anthony S. G., Schipper H. M., Tavares R., Hovanesian V., Cortez S. C., Stopa E. G., and Johanson C. E. (2003) Stress protein expression in the Alzheimer-diseased choroid plexus. J. Alzheimers Dis. 5, 171–177.
Blalock E. M., Geddes J. W., Chen K. C., Porter N. M., Markesbery W. R., and Landfield P. W. (2004) Incipient Alzheimer's disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc. Natl. Acad. Sci. U.S.A. 101, 2173–2178.
Bollag D. M., McQueney P. A., Zhu J., Hensens O., Koupal L., Liesch J., et al. (1995) Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 55, 2325–2333.
Burke W.J., Raghu G., and Strong R. (1994) Taxol protects against calcium-mediated death of differentiated rat pheochromocytoma cells. Life Sci. 55, 313–319.
Busciglio J., Lorenzo A., Yeh J., and Yankner B. A. (1995) Beta-amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron 14, 879–888.
Chapman R., Sidrauski C., and Walter P. (1998) Intracellular signaling from the endoplasmic reticulum to the nucleus. Annu. Rev. Cell Dev. Biol. 14, 459–485.
Darios F., Muriel M. P., Khondiker M. E., Brice A., and Ruberg M. (2005) Neurotoxic calcium transfer from endoplasmic reticulum to mitochondria is regulated by cyclin-dependent kinase 5-dependent phosphorylation of tau. J. Neurosci. 25, 4159–4168.
Divinski I., Mittelman L., and Gozes I. (2004) A femtomolar acting octapeptide interacts with tubulin and protects astrocytes against zinc intoxication. J. Biol. Chem. 279, 28,531–28,538.
Ghribi O., Herman M. M., DeWitt D. A., Forbes M. S., and Savory J. (2001) Abeta(1–42) and aluminum induce stress in the endoplasmic reticulum in rabbit hippocampus, involving nuclear translocation of gad d 153 and NF-kappaB. Brain Res. Mol. Brain Res. 96, 30–38.
Goedert M. (1998) Neurofibrillary pathology of Alzheimer's disease and other tauopathies. Prog. Brain Res. 117, 287–306.
Gozes I. and Divinski I. (2004) The femtomolar-acting NAP interacts with microtubules: novel aspects of astrocyte protection. J. Alzheimers Dis. 6, S37–41.
Grace E. A., Rabiner C. A., and Busciglio J. (2002) Characterization of neuronal dystrophy induced by fibrillar amyloid beta: implications for Alzheimer's disease. Neuroscience 114, 265–273.
Guo Q., Furukawa K., Sopher B. L., Pham D. G., Xie J., Robinson N., et al. (1996) Alzheimer's PS-1 mutation perturbs calcium homeostasis and sensitizes PC12 cells to death induced by amyloid beta-peptide. Neuroreport 8, 379–383.
Guo Q., Sopher B. L., Furukawa K., Pham D. G., Robinson N., Martin G. M., and Mattson M. P. (1997) Alzheimer's presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: involvement of calcium and oxyradicals. J. Neurosci. 17, 4212–4222.
Harding H. P., Zhang Y., and Ron D. (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271–274.
Herms J., Schneider I., Dewachter I., Caluwaerts N., Kretzschmar H., and Van Leuven F. (2003) Capacitive calcium entry is directly attenuated by mutant presenilin-1, independent of the expression of the amyloid precursor protein. J. Biol. Chem. 278, 2484–2489.
Katayama T., Imaizumi K., Honda A., Yoneda T., Kudo T., Takeda M., et al. (2001) Disturbed activation of endoplasmic reticulum stress transducers by familial Alzheimer's disease-linked presenilin-1 mutations. J. Biol. Chem. 276, 43,446–43,454.
Katayama T., Imaizumi K., Manabe T., Hitomi J., Kudo T., and Tohyama M. (2004) Induction of neuronal death by ER stress in Alzheimer's disease. J. Chem. Neuroanat. 28, 67–78.
Kaufman R. J. (1999) Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 13, 1211–1233.
Lee K., Tirasophon W., Shen X., Michalak M., Prywes R., Okada T., et al. (2002) IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 16, 452–466.
Lee V. M., Daughenbaugh R., and Trojanowski J. Q. (1994) Microtubule stabilizing drugs for the treatment of Alzheimer's disease. Neurobiol. Aging 15(Suppl. 2), S87–89.
Leissring M. A., Akbari Y., Fanger C. M., Cahalan M. D., Mattson M. P., and LaFerla F. M. (2000) Capacitative calcium entry deficits and elevated luminal calcium content in mutant presenilin-1 knockin mice. J. Cell Biol. 149, 793–798.
Leissring M. A., Paul B. A., Parker I., Cotman C. W., and LaFerla F. M. (1999) Alzheimer's presenilin-1 mutation potentiates inositol 1,4,5-trisphosphate-mediated calcium signaling in Xenopusoocytes. J. Neurochem. 72, 1061–1068.
Li G., Faibushevich A., Turunen B. J., Yoon S. O., Georg G., Michaelis M. L., and Dobrowsky R. T. (2003) Stabilization of the cyclin-dependent kinase 5 activator, p35, by paclitaxel decreases beta-amyloid toxicity in cortical neurons. J. Neurochem. 84, 347–362.
Mattson M. P. (1997) Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol. Rev. 77, 1081–1132.
Mattson M. P. and Chan S. L. (2001) Dysregulation of cellular calcium homeostasis in Alzheimer's disease: bad genes and bad habits. J. Mol. Neurosci. 17, 205–224.
Mattson M. P., Chan S. L., and Camandola S. (2001) Presenilin mutations and calcium signaling defects in the nervous and immune systems. Bioessays 23, 733–744.
Mattson M. P., Mark R. J., Furukawa K., and Bruce A. J. (1997) Disruption of brain cell ion homeostasis in Alzheimer's disease by oxy radicals, and signaling pathways that protect therefrom. Chem. Res. Toxicol. 10, 507–517.
Michaelis M. L., Ansar S., Chen Y., Reiff E. R., Seyb K. I., Himes R. H., et al. (2005) β-amyloid-induced neurodegeneration and protection by structurally diverse microtubule-stabilizing agents. J. Pharmacol. Exp. Ther. 312, 659–668.
Michaelis M. L., Dobrowsky R. T., and Li G. (2002) Tau neurofibrillary pathology and microtubule stability. J. Mol. Neurosci. 19, 289–293.
Michaelis M. L., Ranciat N., Chen Y., Bechtel M., Ragan R., Hepperle M., et al. (1998) Protection against beta-amyloid toxicity in primary neurons by paclitaxel (Taxol). J. Neurochem. 70, 1623–1627.
Morishima N., Nakanishi K., Takenouchi H., Shibata T., and Yasuhiko Y. (2002) An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J. Biol. Chem. 277, 34,287–34,294.
Noble P. and Mayer-Proschel M. (1998) Culture of astocytes, oligodendrocytes and O-2A progenitor cells, in Culturing Nerve Cells, 2nd Edition (Banker G. and Goslin K., eds) MITT Press, Cambridge, MA, pp 499–543.
Pal R., Agbas A., Bao X., Hui D., Leary C., Hunt J., et al. (2003). Selective dendrite-targeting of mRNAs of NR1 splice variants without exon 5: identification of a cisacting sequence and isolation of sequence-binding proteins. Brain Res. 994, 1–18.
Pestova T. V., Kolupaeva V. G., Lomakin I. B., Pilipenko E. V., Shatsky I. N., Agol V. I., and hellen C. U. (2001) Molecular mechanisms of translation initiation in eukaryotes. Proc. Natl. Acad. Sci. U.S.A. 98, 7029–7036.
Rao R. V., Castro-Obregon S., Frankowski H., Schuler M., Stoka V., del Rio G., et al. (2002) Coupling endoplasmicreticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J. Biol. Chem. 277, 21,836–21,842.
Ron D. (2002) Translational control in the endoplasmic reticulum stress response. J. Clin. Invest. 110, 1383–1388.
Selkoe D. J. (2001) Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 81, 741–766.
Sherman M. Y. and Goldberg A. L. (2001) Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron 29, 15–32.
Siman R., Flood D. G., Thinakaran G., and Neumar R. W. (2001) Endoplasmic reticulum stress-induced cysteine protease activation in cortical neurons: effect of an Alzheimer's disease-linked presenilin-1 knock-in mutation. J. Biol. Chem. 276, 44,736–44,743.
Song L., De Sarno P., and Jope R. S. (2002) Central role of glycogen synthase kinase-3beta in endoplasmic reticulum stress-induced caspase-3 activation. J. Biol. Chem. 277, 44,701–44,708.
Sood R., Porter A. C., Ma K., Quilliam L. A., and Wek R. C. (2000) Pancreatic eukaryotic initiation factor-2alpha kinase (PEK) homologues in humans, Drosophila melanogaster and Caenorhabditis elegans that mediate translational control in response to endoplasmic reticulum stress. Biochem. J. 346(Pt. 2), 281–293.
Spillantini M. G. and Goedert M. (1998) Tau protein pathology in neurodegenerative diseases. Trends Neurosci. 21, 428–433.
Sponne I., Fifre A., Drouet B., Klein C., Koziel V., Pincon-Raymond M., et al. (2003) Apoptotic neuronal cell death induced by the non-fibrillar amyloid-beta peptide proceeds through an early reactive oxygen species-dependent cytoskeleton perturbation. J. Biol. Chem. 278, 3437–3445.
Suen K. C., Yu M. S., So K. F., Chang R. C., and Hugon J. (2003) Upstream signaling pathways leading to the activation of double-stranded RNA-dependent serine/threonine protein kinase in beta-amyloid peptide neurotoxicity. J. Biol. Chem. 278, 49,819–49,827.
Terro F., Czech C., Esclaire F., Elyaman W., Yardin C., Baclet M. C., et al. (2002) Neurons overexpressing mutant presenilin-1 are more sensitive to apoptosis induced by endoplasmic reticulum-Golgi stress. J. Neurosci. Res. 69, 530–539.
Tirasophon W., Lee K., Callaghan B., Welihinda A., and Kaufman R. J. (2000) The endoribonuclease activity of mammalian IRE1 autoregulates its mRNA and is required for the unfolded protein response. Genes Dev. 14, 2725–2736.
Travers K. J., Patil C. K., Wodicka L., Lockhart D. J., Weissman J. S., and Walter P. (2000) Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101, 249–258.
Trushina E., Heldebrant M. P., Perez-Terzic C. M., Bortolon R., Kovtun I. V., Badger J. D. 2nd, et al. (2003) Microtubule destabilization and nuclear entry are sequential steps leading to toxicity in Huntington's disease. Proc. Natl. Acad. Sci. U.S.A. 100, 12,171–12,176.
Zaidi A. and Michaelis M. L. (1999) Effects of reactive oxygen species on brain synaptic plasma membrane Ca(2+)-ATPase. Free Radic. Biol. Med. 27, 810–821.
Zaidi A., Barron L., Sharov V.S., Schoneich C., Michaelis E. K., and Michaelis M. L. (2003) Oxidative inactivation of purified plasma membrane Ca2+-ATPase by hydrogen peroxide and protection by calmodulin. Biochemistry 42, 12,001–12,010.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seyb, K.I., Ansar, S., Bean, J. et al. β-amyloid and endoplasmic reticulum stress reponses in primary neurons. J Mol Neurosci 28, 111–123 (2006). https://doi.org/10.1385/JMN:28:2:111
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:28:2:111