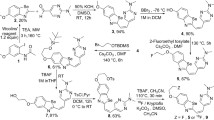
Abstract
Extensive deposition of neuritic and diffuse amyloid plaques in the brain is a critical event for the pathogenesis of Alzheimer’s disease (AD) and considered to start before the appearance of clinical symptoms. In vivo detection of these brain β-amyloid (Aβ) deposits using positron emission tomography (PET), therefore, would be a useful marker for presymptomatic detection of AD. To develop a new agent for PET probe of imaging neuritic and diffuse amyloid deposits, novel fluorescent compounds, including styryl-fluorobenzoxazole derivatives, were examined. These compounds showed a high binding affinity for both synthetic Aβ1-40 and Aβ1-42 aggregates. Some of these compounds also displayed distinct staining of neuritic and diffuse amyloid plaques in AD brain sections. A biodistribution study of styryl-fluorobenzoxazole derivatives in normal mice exhibited excellent brain uptakes (4.5–5.5% injected dose/g at 2 min postinjection). Furthermore, iv administration of BF-145, a styryl-fluorobenzoxazole derivative, demonstrated specific in vivo labeling of compact and diffuse amyloid deposits in an APP23 transgenic mouse brain, in contrast to no accumulation in a wild-type mouse brain. These findings suggest that BF-145 is a potential candidate as a probe for imaging early brain pathology in AD patients.
Similar content being viewed by others
References
Agdeppa E. D., Kepe V., Liu J., Flores-Torres S., Satyamurthy N., Petric A., et al. (2001) Binding characteristics of radiofluorinated 6-dialkylamino-2-naphthylethylidene derivatives as positron emission tomography imaging probes for beta-amyloid plaques in Alzheimer’s disease. J. Neurosci. 21, RC189.
Bacskai B. J., Kajdasz S. T., Christie R. H., Carter C., Games D., Seubert P., et al. (2001) Imaging of amyloid-beta deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat. Med. 7, 369–372.
Bacskai B. J., Klunk W. E., Mathis C. A., and Hyman B. T. (2002) Imaging amyloid-β deposits in vivo. J. Cereb. Blood Flow Metab. 22, 1035–1041.
Cheng Y. and Prusoff W. H. (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (150) of an enzymatic reaction. Biochem. Pharmacol. 22, 3099–3108.
Crystal A. S., Giasson B. I., Crowe A., Kung M. P., Zhuang Z. P., and Trojanowski J. Q. (2003) A comparison of amyloid fibrillogenesis using the novel fluorescent compound K114. J. Neurochem. 86, 1359–1368.
Du Y., Wei X., Dodel R., Sommer N., Hampel H., Gao F., et al. (2003) Human anti-β-amyloid antibodies block β-amyloid fibril formation and prevent β-amyloid-induced neurotoxicity. Brain 126, 1935–1939.
Engler H., Blomqvist G., Bergstrom M., Langstrom B., Klunk W., Debnath M., et al. (2002) First human study with a benzothiazole amyloid-imaging agent in Alzheimer’s disease and control subjects. Neurobiol. Aging 23, S1568.
Hardy J. and Selkoe D. J. (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356.
Helmuth L. (2002) New Alzheimer’s treatments that may ease the mind. Science 297, 1260–1262.
Kitamoto T., Ogomori K., Tateishi J., and Prusiner S. B. (1987) Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab. Invest. 57, 230–236.
Klunk W. E., Bacskai B. J., Mathis C. A., Kajdasz S. T., McLellan M. E., Frosch M. P., et al. (2002) Imaging Abeta plaques in living transgenic mice with multiphoton microscopy and methoxy-X04, a systemically administered Congo red derivative. J. Neuropathol. Exp. Neurol. 61, 797–805.
Klunk W. E., Wang Y., Huang G. F., Debnath M. L., Holt D. P., Shao L., et al. (2003) The binding of 2-(4′-methylaminophenyl)benzothiazole to postmortem brain homogenates is dominated by the amyloid component. J. Neurosci. 23, 2086–2092.
Kung H. F., Kung M. P., Zhuang Z. P., Hou C., Lee C. W. Plossl K., et al. (2003) Iodinated tracers for imaging amyloid plaques in the brain. Mol. Imaging Biol. 5, 418–426.
Kung M. P., Hou C., Zhuang Z. P., Zhang B., Skovronsky D., Trojanowski J. Q., et al. (2002) IMPY: an improved thioflavin-T derivative for in vivo labeling of beta-amyloid plaques. Brain Res. 956, 202–210.
Kung M. P., Skovronsky D. M., Hou C., Zhuang Z.P., Gur T. L., Zhang B., et al. (2003a) Detection of amyloid plaques by radioligands for Abeta40 and Abeta42: potential imaging agents in Alzheimer’s patients. J. Mol. Neurosci. 20, 15–24.
Kung M. P., Zhuang Z. P., Hou C., Jin L. W., and Kung H. F. (2003b) Characterization of radioiodinated ligand binding to amyloid beta plaques. J. Mol. Neurosci. 20, 249–254.
Lee C. W., Kung M. P., Hou C., and Kung H. F. (2003) Dimethylamino-fluorenes: ligands for detecting beta-amyloid plaques in the brain. Nucl. Med. Biol. 30, 573–580.
Mathis C. A., Bacskai B. J., Kajdasz S. T., McLellan M. E., Frosch M. P., Hyman B. T., et al. (2002) A lipophilic thioflavin-T derivative for positron emission tomography (PET) imaging of amyloid in brain. Bioorg. Med. Chem. Lett. 12, 295–298.
Morris J. C., Storandt M., McKeel D. W., Jr., Rubin E. H., Price J. L., Grant E. A., et al. (1996) Cerebral amyloid deposition and diffuse plaques in “normal” aging: Evidence for presymptomatic and very mild Alzheimer’s disease. Neurology 46, 707–719.
Ono M., Wilson A., Nobrega J., Westaway D., Verhoeff P., Zhuang Z. P., et al. (2003) 11C-Labeled stilbene derivatives as Aβ-aggregate-specific PET imaging agents for Alzheimer’s disease. Nucl. Med. Biol. 30, 565–571.
Price J. L. and Morris J. C. (1999) Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann. Neurol. 45, 358–368.
Selkoe D. J. (2000) Imaging Alzheimer’s amyloid. Nat. Biotechnol. 18, 823, 824.
Shoghi-Jadid K., Small G. W., Agdeppa E. D., Kepe V., Ercoli L. M., Siddarth P., et al. (2002) Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer disease. Am. J. Geriatr. Psychiatry 10, 24–35.
Skovronsky D. M., Zhang B., Kung M. P., Kung H. F., Trojanowski J. Q., and Lee V. M. (2000) In vivo detection of amyloid plaques in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 97, 7609–7614.
Small G. W., Agdeppa E. D., Kepe V., Satyamurthy N., Huang S.-C., and Barrio J. R. (2002) In vivo brain imaging of tangle burden in humans. J. Mol. Neurosci. 19, 323–327.
Sturchler-Pierrat C., Abramowski D., Duke M., Wiederhold K. H., Mistl C., Rothacher S., et al. (1997) Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl. Acad. Sci. U. S. A. 94, 13287–13292.
Suemoto T., Okamura N., Shiomitsu T., Suzuki M., Shimadzu H., Akatsu H., et al. (2004) In vivo amyloid-β labeling in a model mouse with BF-108. Neurosci. Res. 48, 65–74.
Trojanowski J. Q. (2002) Emerging Alzheimer’s disease therapies: focusing on the future. Neurobiol. Aging 23, 85–990.
Vickers J. C., Dickson T. C., Adlard P. A., Saunders H. L., King C. E., and McCormack G. (2000) The cause of neuronal degeneration in Alzheimer’s disease. Prog. Neurobiol. 60, 139–165.
Wu C. W., Liao P. C., Lin C., Kuo C. J., Chen S. T., Chen H. I., et al. (2003) Brain region-dependent increases in beta-amyloid and apolipoprotein E levels in hypercholesterolemic rabbits. J. Neural. Transm. 110, 641–649.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okamura, N., Suemoto, T., Shiomitsu, T. et al. A novel imaging probe for in vivo detection of neuritic and diffuse amyloid plaques in the brain. J Mol Neurosci 24, 247–255 (2004). https://doi.org/10.1385/JMN:24:2:247
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:24:2:247