Abstract
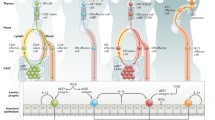
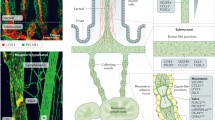
Lymphoid organogenesis is dependent upon a series of intricate cellular interactions involving adhesion molecules, chemokines, and cytokines that generate fully compartmentalized lymphoid structures. Development of organized lymphoid structures in the intestine begins prenatally and continues through adulthood, with constant adaptations to changes in the luminal flora. While much is known about the mechanisms that govern the development of macroscopic intestinal lymphoid structures (mesenteric lymph node and Peyer's patches), the organogenesis of the microscopic lymphoid tissues (including cryptopatches and isolated lymphoid follicles) is an emerging field. This review examines the current state of knowledge about organogenesis of the known types of organized lymphoid tissue in the intestine and identifies unique and common features in the development of each of the structures discussed.
Similar content being viewed by others
References
Wigle JT, Oliver G: Prox1 function is required for the development of the murine lymphatic system. Cell 1999; 98:769–778.
Wigle JT, Harvey N, Detmar M, et al: An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J 2002;21:1505–1513.
DeTogni P, Goellner J, Ruddle NH, et al.: Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 1994;264:703–707.
Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA: Distinct roles in lymphoid organo genesis for lymphotoxins α and β revealed in lymphotoxin β-deficient mice. Immunity 1997;6:491–500.
Honda K, Nakano H, Yoshida H, et al.: Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer's patch organogenesis. J Exp Med 2001; 193:621–630.
Mebius RE, Rennert P, Weissman IL: Developing lymph nodes collect CD4+CD3-LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 1997;7:493–504.
Yoshida H, Honda K, Shinkura R, et al.: IL-7 receptor α+ CD3− cells in the embryonic intestine induces the organizing center of Peyer's patches. Int Immunol 1999; 11:643–655.
Eberl G, Littman DR: The role of the nuclear hormone receptor RORψt in the development of lymph nodes and Peyer's patches. Immunol Rev 2003;195:81–90.
Sun Z, Unutmaz D, Zou YR, et al.: Requirement for RORψ in thymocyte survival and lymphoid organ development. Science 2000;288:2369–2373.
Mauri DN, Ebner R, Montgomery RI, et al.: LIGHT, a new member of the TNF superfamily, and lymphotoxin a are ligands for herpesvirus entry mediator. Immunity 1998;8:21–30.
Cohavy O, Zhou J, Ware CF, Targan SR: LIGHT is constitutively expressed on T and NK cells in the human gut and can be induced by CD2-mediated signaling. J Immunol 2005;174:646–653.
Scheu S, Alferink J, Potzel T, Barchet W, Kalinke U, Pfeffer K: Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin β in mesenteric lymph node genesis. J Exp Med 2002;195:1613–1624.
Yoshida H, Naito A, Inoue J, et al.: Different cytokines induce surface lymphotoxin-αβ on IL-7 receptor-α cells that differentially engender lymph nodes and Peyer's patches. Immunity 2002;17:823–833.
Muller JR, Siebenlist U: Lymphotoxin β receptor induces sequential activation of distinct NF-κB factors via separate signaling pathways. J Biol Chem 2003; 278:12006–12012.
Dejardin E, Droin NM, Delhase M, et al.: The lymphotoxin-β receptor induces different patterns of gene expression via two NF-κB pathways. Immunity 2002; 17:525–535.
Ngo VN, Korner H, Gunn MD, et al.: Lymphotoxin α/β and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med 1999;189:403–412.
Kim D, Mebius RE, MacMicking JD, et al.: Regulation of peripheral lymph node genesis by the tumor necrosis factor family member TRANCE. J Exp Med 2000; 192:1467–1478.
Mebius RE: Organogenesis of lymphoid tissues. Nat Rev Immunol 2003;3:292–303.
Luther SA, Ansel KM, Cyster JG: Overlapping roles of CXCL13, interleukin 7 receptor α, and CCR7 ligands in lymph node development. J Exp Med 2003;197: 1191–1198.
Ansel KM, Ngo VN, Hyman PL, et al.: A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 2000;406:309–314.
Cao X, Shores EW., Hu-Li J, et al.: Defective lymphoid development in mice lacking expression of the common cytokine receptor γ chain. Immunity 1995; 2:223–238.
Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M: A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 1996;87: 1037–1047.
Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS: Surface lymphotoxin α/β complex is required for the development of peripheral lymphoid organs. J Exp Med 1996;184:1999–2006.
Cupedo T, Vondenhoff MF, Heeregrave EJ, et al.: Presumptive lymph node organizers are differentially represented in developing mesenteric and peripheral nodes. J Immunol 2004;173:2968–2975.
Adachi S, Yoshida H, Kataoka H, Nishikawa S: Three distinctive steps in Peyer's patch formation of murine embryo. Int Immunol 1997;9:507–514.
Nishikawa S, Honda K, Vieira P, Yoshida H: Organogenesis of peripheral lymphoid organs. Immunol Rev 2003;195:72–80.
Dougall WC, Glaccum M, Charrier K, et al.: RANK is essential for osteoclast and lymph node development. Genes Dev 1999;13:2412–2424.
Sims JE, Williams DE, Morrissey PJ, et al.: Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J Exp Med 2000; 192:671–680.
Pandey A, Ozaki K, Baumann H, et al.: Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol 2000;1:59–64.
Park LS, Martin U, Garka K, et al.: Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med 2000;192:659–670.
Mebius RE, Streeter PR, Michie S, Butcher EC, Weissman IL: A developmental switch in lymphocyte homing receptor and endothelial vascular addressin expression regulates lymphocyte homing and permits CD4+CD3−cells to colonize lymph nodes. Proc Natl Acad Sci USA 1996;93:11019–11024.
Finke D, Acha-Orbea H, Mattis A, Lipp M, Kraehenbuhl J: CD4+CD3− cells induce Peyer's patch development: role of α4β1 integrin activation by CXCR5. Immunity 2002;17:363–373.
Hashi H, Yoshida H, Honda K, et al.: Compartmentalization of Peyer's patch anlagen before lymphocyte entry. J Immunol 2001;166:3702–3709.
Tumanov AV, Kuprash DV, Mach JA, Nedospasov SA, Chervonsky AV: Lymphotoxin and TNF produced by B cells are dispensable for maintenance of the follicle-associated epithelium but are required for development of lymphoid follicles in the Peyer's patches. J Immunol 2004;173:86–91.
Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG: Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci USA 2000;97:12694–12699.
Forster R, Schubel A, Breitfeld D, et al.: CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 1999;99:23–33.
Bastlein C, Burlefinger R, Holzberg E, Voeth C, Garbrecht M, Ottenjann R: Common variable immunodeficiency syndrome and nodular lymphoid hyperplasia in the small intestine. Endoscopy 1988;20:272–275.
Hamada H, Hiroi T, Nishiyama Y, et al.: Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol 2002; 168:57–64.
Lorenz RG, Newberry RD: Isolated lymphoid follicies can function as sites for induction of mucosal immune responses. Ann NY Acad Sci 2004;1029:44–57.
Yamamoto M, Rennert P, McGhee JR, et al.: Alternate mucosal immune system: organized Peyer's patches are not required for IgA responses in the gastrointestinal tract. J Immunol 2000;164:5184–5191.
Lorenz RG, Chaplin DD, McDonald KG, McDonough JS, Newberry RD: Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin β receptor, and TNF receptor I function. J Immunol 2003;170:5475–5482.
Hjelmstrom P: Lymphoid neogenesis: denovo formation of lymphoid tissue in chronic inflammation through expression of homing chemokines. J Leukoc Biol 2001; 69:331–339.
Grant AJ, Goddard S, Ahmed-Choudhury J, et al.: Hepatic expression of secondary lymphoid chemokine (CCL21) promotes the development of portal-associated lymphoid tissue in chronic inflammatory liver disease. Am J Pathol 2002;160:1445–1455.
Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T: Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science 2002;298: 1424–1427.
Suzuki K, Meek B, Doi Y, et al.: Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci USA 2004;101:1981–1986.
Kanamori Y, Ishimaru K, Nanno M, et al.: Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+IL-7R+Thyl+ lympho-hemopoietic progenitors develop. J Exp Med 1996;184:1449–1459.
Taylor RT, Lugering A, Newell KA, Williams IR: Intestinal cryptopatch formation in mice requires lymphotoxin α and the lymphotoxin β receptor. J Immunol 2004; 173:7183–7189.
Shinkura R, Kitada K, Matsuda F, et al.: Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-κb-inducing kinase. Nat Genet 1999; 22:74–77.
Macpherson AJ, Uhr T: The donor splice site mutation in NFκB-inducing kinase of alymphoplasia (aly/aly) mice. Immunogenetics 2003;54:693–698.
Yin L, Wu L, Wesche H, et al.: Defective lymphotoxin-β receptor-induced NF-κB transcriptional activity in NIK-deficient mice. Science 2001;291:2162–2165.
Oida T, Suzuki K, Nanno M, et al.: Role of gut cryptopatches in early extrathymic maturation of intestinal intraepithelial T cells. J Immunol 2000;164:3616–3626.
Saito H, Kanamori Y, Takemori T, et al.: Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science 1998;280:275–278.
Suzuki K, Oida T, Hamada H, et al.: Gut cryptopatches: direct evidence of extrathymic anatomical sites for intestinal T lymphopoiesis. Immunity 2000;13:691–702.
Rocha B, Vassalli P, Guy-Grand D: Thymic and extrathymic origins of gut intraepithelial lymphocyte populations in mice. J Exp Med 1994;180:681–686.
Lambolez F, Azogui O, Joret AM, et al.: Characterization of T cell differentiation in the murine gut. J Exp Med 2002;195:437–449.
Pabst O, Herbrand H, Worbs T, et al.: Cryptopatches and isolated lymphoid follicles: dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur J Immunol 2005;35:98–107.
Guy-Grand D, Azogui O, Celli S, et al.: Extrathymic T cell lymphopoiesis: ontogeny and contribution to gut intraepithelial lymphocytes in athymic and euthymic mice. J Exp Med 2003;197:333–341.
Nonaka S, Naito T, Chen H, et al.: Intestinal ψδT Cells develop in mice lacking thymus, all lymph nodes. Peyer's patches, and isolated lymphoid follicles. J Immunol 2005;174:1906–1912.
Eberl G, Littman DR: Thymic origin of intestinal αβ T Cells revealed by fate mapping of RORγt+ cells. Science 2004;305:248–251.
Mayrhofer G, Moghaddami M, Murphy C: Lymphocyte-filled villi (LFV): non-classical organized lymphoid tissues in the mucosa of the small intestine. Mucosal Immunology Update 1999;7:9–13.
Mayrhofer G, Brooks A: Lymphopoeisis in lymphocyte-filled villi in the small intestine of the rat. Clin Immunol Immunopathol 1995;76:S55.
Moghaddami M, Cummins A, Mayrhofer G: Lymphocyte-filled villi: comparison with other lymphoid aggregations in the mucosa of the human small intestine. Gastroenterology 1998;115:1414–1425.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taylor, R.T., Williams, I.R. Lymphoid organogenesis in the intestine. Immunol Res 33, 167–181 (2005). https://doi.org/10.1385/IR:33:2:167
Issue Date:
DOI: https://doi.org/10.1385/IR:33:2:167