Abstract
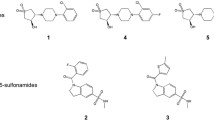
The development of new therapies against infectious diseases is vital in developing countries. Among infectious diseases, tuberculosis is considered the leading cause of death. A target for development of new drugs is the tryptophan pathway. The last enzyme of this pathway, tryptophan synthase (TRPS), is responsible for conversion of the indole 3-glycerol phosphate into indol and the condensation of this molecule with serine-producting tryptophan. The present work describes the molecular models of TRPS from Mycobacterium tuberculosis (MtTRPS) complexed with six inhibitors, the indole 3-propanol phosphate and five arylthioalkyl-phosphonated analogs of substrate of the α-subunit. The molecular models of MtTRPS present good stereochemistry, and the binding of the inhibitors is favorable. Thus, the generated models can be used in the design of more specific drugs against tuberculosis and other infectious diseases.
Similar content being viewed by others
References
Trouiller, P., Torreele, E., Olliaro, P., et al. (2001) Drugs for neglected diseases: a failure of the market and a public health failure? Trop. Med. Int. Health 6, 945–951.
World Health Organization. Global Tuberculosis Control. WHO Report 2001. Geneva, Switzerland, WHO/CDS/TB/2001.287
Dosselaere, F. and Vanderleyden, J. (2001) A metabolic node in action: chorismate-utilizing enzymes in microorganisms. Crit. Rev. Microbiol. 27, 75–131.
Roberts, F., Roberts, C. W., Johnson, J. J., et al. (1998) Evidence for the shikimate pathway in apicomplexan parasites. Nature 393, 801–805.
Schonbrunn, E., Eschenburg, S., Shuttleworth, W. A., et al., (2001) Interaction of the herbicide glyphosate with its target enzyme 5-enolpyruvylshikimate 3-phosphate synthase in atomic detail. Proc. Natl. Acad. Sci. U.S.A. 98, 1376–1380.
Daves, G. M., Barrett-Bee, K. J. Jude, D. A., et al. (1994) (6S)-6-Fluoroshikimic acid, an antibacterial agent acting on the aromatic biosynthetic pathway. Antimicrob. Agents Chemother. 38, 403–406.
Finn, J., Langevine, C., Birk, I., Nicherson, K., and Rodaway, S. (1999) Rational herbicide design by inhibition of tryptophan biosynthesis. Bioorg. Med. Chem. Lett. 9, 2297–2302.
Smith, D. A., Parish, T., Stoker, N. G., and Bancroft, G. J. (2001) Characterization of auxotrophic mutants of Mycobacterium tuberculosis and their potential as vaccine candidates. Infect. Immun. 69, 1142–1150.
Hyde, C. C. and Miles, E. W. (1990) The tryptophan synthase multienzyme complex: exploring structure-function relationships with X-ray crystallography and mutagenesis. Biotechnology 8, 27–32.
Pan, P., Woehl, E., and Dunn, M. F. (1997) Protein architecture, dynamics and allostery in trytophan synthase channeling. Trends Biochem. Sci. 22, 22–27.
Weyand, M. and Schlichting, I. (1999) Crystal structure of wild-type tryptophan synthase complexed with the natural substrate indole-3-glycerol phosphate. Biochemistry 38, 16,469–16,480.
Sachpatzidis, A., Dealwis, C., Lubetsky, J. B., Liang, P. H., Anderson, K. S., and Lolis, E. (1999) Crystallographic studies of phosphonate-based alpha-reaction transition-state analogues complexed to tryptophan synthase. Biochemistry 38, 12,665–12,674.
Uchoa, H. B., Jorge, G. E., da Silveira, N. J. F., Camera, J. C., Jr., Canduri, F., and de Azevedo, W. F., Jr. (2004) Parmodel: a web server for automated comparative modeling of proteins. Biochem. Biophys. Res. Commun. 325, 1481–1486.
Sali, A. and Blundell, T. L. (1993) Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815.
Collaborative Computational Project No. 4 (1994) The CCP4 suite: program for protein crystallography. Acta Crystallogr. D 50, 760–763.
Brünger, A. T. (1992) X-PLOR version 3.1: a system for crystallography and NMR. Yale University Press, New Haven, CT.
Bowie, J. U., Luthy, R., and Eisenberg, D. (1991) A method to identify protein sequences that fold into a known three-dimensional structure. Science 253, 164–170.
Luthy, R., Bowie, J., and Eisenberg D. (1992) Assessment of protein models with three-dimensional profiles. Nature 356, 83–85.
Wang, R., Liu, L., Lai, L., and Tang, Y. (1998) SCORE: a new empirical method for estimating the binding affinity of a protein-ligand complex. J. Mol. Model. 4, 379–394.
Hyde, C. C., Ahmed, S. A., Padlan, E. A., Miles, E. W., and Davies, D. R. (1988) Three-dimensional structure of the tryptophan synthase α2β2 multienzyme complex from Salmonella typhimurium. J. Biol. Chem. 263, 17,857–17,871.
Banner, D. W., Bloomer, A. C., Petsko, G. A., et al. (1975) Structure of chicken muscle triose phosphate isomerase determined crystallographically at 2.5 Å resolution using amino acid sequence data. Nature 255, 609–614.
Rhee, S., Miles, E. W., Mozzarelli, A., and Davies, D. R. (1998) Cryocrystallography and microspectrophotometry of a mutant (alpha D60N) tryptophan synthase alpha 2 beta 2 complex reveals allosteric roles of alpha Asp60. Biochemistry 37, 10,653–10,659.
De Azevedo, W. F., Jr., Mueller-Dieckmann, H. J., Schulze-Gahmen, U., Worland, P. J., Sausville, E., and Kim, S. H. (1996) Structural basis for specificity and potency of a flavonoid inhibitor of human CDK2, a cell cycle kinase. Proc. Natl. Acad. Sci. U.S.A. 93, 2735–2740.
De Azevedo, W. F., Jr., Canduri, F., Dos Santos, D. M., et al. (2003) Structural basis for inhibition of human PNP by immucillin-H. Biochem. Biophys. Res. Commun. 309, 922–927.
Pereira, J. H., Canduri, F., de Oliveira, J. S., et al. (2003) Structural bioinformatics study of EPSP synthase from Mycobacterium tuberculosis. Biochem. Biophys. Res. Commun. 312, 608–614.
Thompson, J. D., Higgins, D. G., and Gibson, T. J. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680.
Koradi, R., Billeter, M., and Wüthrich, K. (1996) MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graph. 14, 51–55.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Dias, M.V.B., Canduri, F., da Silveira, N.J.F. et al. Molecular models of tryptophan synthase from Mycobacterium tuberculosis complexed with inhibitors. Cell Biochem Biophys 44, 375–384 (2006). https://doi.org/10.1385/CBB:44:3:375
Issue Date:
DOI: https://doi.org/10.1385/CBB:44:3:375