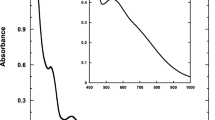
Abstract
The interaction of sodium metavanadate and VOCl3 with ascorbic acid, one of the possible natural reducing agents of vanadium(V) to oxovanadium(IV), has been investigated. Three new VO2+ complexes could be isolated as microcrystalline powders. One of them, of composition K1.5Na0.5[VO(HAsc)(OH)3], contains ascorbic acid as a monodentate ligand. In the other two, K[VO(Diketo)(OH)]·H2O and Na3[VO(Diketo)2(OH)], the enolized form of 2,3-diketogulonic acid (one of the oxidation products of ascorbic acid), acts as a bidentate ligand. The complexes were characterized by means of electronic (absorption and reflectance) and infrared spectroscopy and magnetic susceptibility measurements. Their thermal behavior was investigated by thermogravimetric and differential thermal analyses. The interest of the investigated system in relation to vanadium detoxification is also discussed.
Similar content being viewed by others
References
E. J. Baran, Model studies related to vanadium metabolism, Bol. Soc. Chil. Quím. 42, 247–256 (1997).
E. J. Baran, Oxovanadium (IV) and oxovanadium (V) complexes relevant to biological systems, J. Inorg. Biochem. 80, 1–10 (2000).
E. J. Baran, Vanadium detoxification, in Vanadium in the Environment, J. O. Nriagau, ed., Wiley, New York, Part 2, pp.317–345 (1998).
M. Ding, P. M. Gannett, Y. Rojanasakul, K. Liu, and X. Shi, One-electron reduction of vanadate by ascorbate and related free radical generation at physiological pH, J. Inorg. Biochem. 55, 101–112 (1994).
E. G. Ferrer, P. A. M. Williams, and E. J. Baran, Interaction of the vanadyl(IV) cation with l-ascorbic acid and related systems, Z. Naturforsch. 53b, 256–262 (1998).
M. M. Jones and M. A. Basinger, Chelate antidotes for sodium vanadate and vanadyl sulfate intoxication in mice, J. Toxicol. Environ. Health 12, 749–756 (1983).
H. Onishi, Photometric Determination of Traces of Metals, 4th ed., J Wiley, New York, Part IIB, pp. 674–676 (1989).
H. G. Seiler, A. Sigel, and H. Sigel, eds., Handbook on Metals in Clinical and Analytical Chemistry, Marcel Dekker, New York, pp. 531 and 573 (1994).
A. Syamal, Spin-spin coupling in oxovanadium (IV) complexes, Coord. Chem. Rev. 16, 309–339 (1975).
E. J. Baran, Spectroscopic studies of oxovanadium coordination compounds, J. Coord. Chem., in press.
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, 3rd ed., Wiley, New York (1978).
M. Cieslak-Golonka, M. Raczko, D. Lasut, and I. Maslanka, Electronic and infrared spectra of chromium (III) complexes isolated from the bioinorganic system: [Cr(VI)-ascorbic acid]; Cr(VI)=K2Cr2O7, KCrO3Cl, CrO2Cl2, Spectrochim. Acta 55A, 421–429 (1999).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ferrer, E.G., Baran, E.J. Reduction of vanadium(V) with ascorbic acid and isolation of the generated oxovanadium(IV) species. Biol Trace Elem Res 83, 111–119 (2001). https://doi.org/10.1385/BTER:83:2:111
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/BTER:83:2:111