Abstract
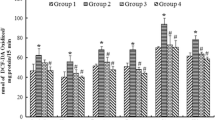
A study was conducted to analyze the regional distribution of Ca, Cu, Fe, Mg, and Zn contents in brain tissues after animals were given liquor of brick tea that contained a high Al content. In 25 normal adult male mice given either water or 0.9% NaCl for 1 mo or 2 mo, the metal concentrations in the serum, liver, frontal cortex, hippocampus, cerebellum, and brain stem were comparable (p>0.05). When the drinking water was replaced by a 1% brick tea liquor, which contained a high Al content, serum Al concentration was increased significantly 1 mo after the onset of the experiment and remained high at the end of the second month. The level of Al was also elevated in both the cortex and hippocampus at 1 mo after replacing tea for drinking water. In addition to Al, there were a significant increase in hippocampal Zn and a decrease in Cu contents. There was no change in tissue Mg or Fe contents, but there was a significant increase in Ca content in every brain region studied. It was suggested that the increase in Ca might be the result of the effect of other components in tea. Unlike the brain, there was no change in the concentration of any of the metals, including Al, in the liver, which further demonstrated that the changes observed in the brain was specific. The results of the present study confirmed that Al, when given orally in the form of tea, could be absorbed into the bloodstream. The absorbed Al could accumulate in selected brain regions. The presence of Al might also change the tissue content of endogenous trace metals.
Similar content being viewed by others
References
J. Walton, G. Hams, and D. Wilcox, Bioabailability of aluminum from drinking water: co-exposure with food beverages, Urban Water Res. Assoc. of Australia Res. Rep. #83 (1994).
D. R. C. McLachlan, Aluminum and the risk for Alzheimer’s disease, Environmentrics 6, 233–275 (1995).
A. M. Coriat and R. D. Gillard, Beware the cup that we share, Nature (London) 321, 570 (1989).
P. O. Owuor, F. O. Gone, D. B. Onchiri, and I. O. Jumba, Levels of aluminum in green leaf of clonal teas, black tea and black tea liquors, and effects of rates of nitrogen fertilizers on the aluminum contents in black tea, Food Chem. 35, 59–68 (1990).
E. M. Chenery, A preliminary study of aluminum and the tea bush, Plant Soil 6, 174–200 (1955).
M. H. Wong, Z. Q. Zhang, C. Y. Lan, and J. W. C. Wong, Trace metal contents (Al, Cu and Zn) of tea: tea and soil from two tea-plantations, and tea products from different provinces of China, Environ. Geochem. Health 20, 87–94 (1998).
F. Santos, J. C. M. Chan, M. S. Yang, J. Savory, and M. R. Wills, Aluminum deposition in the central nervous system, preferential accumulation in the hippocampus in weanling rats, Med. Biol. 65, 53–55 (1987).
M. F. Van Ginkel, G. B. Van der Voet, P. C. D’Haese, M. E. DeBroe, and F. A. DeWolff, Effect of citric acid and maltol on the accumulation of aluminum in rat brain and bone, J. Lab. Clin. Med. 121, 453–460 (1993).
G. J. Fosmire, S. J. Focht, and G. E. McClearn, Genetic influences on tissue deposition of aluminum in mice, Biol. Trace Element Res. 37, 115–121 (1993).
G. Sahin, T. Varol, A. Temizer, K. Benli, R. Demirdamar, and S. Duru, Determination of aluminum levels in the kidney, liver and brain of mice treated with aluminum hydroxide, Biol. Trace Element Res. 41, 129–135 (1994).
M. S. Yang, H. F. Wong, and K. L. Yung, Determination of endogenous trace metal contents in various mouse brain regions after prolonged oral administration of aluminum chloride, J. Toxicol. Environ. Health 55, 445–453 (1998).
W. R. Markesbery, Oxidative stress hypothesis in Alzheimer’s disease, Free Radical Biol. Med. 23, 134–147 (1997).
G. Danscher, K. B. Jensen, C. J. Frederickson, K. Kemp, A. Andreasen, S. Juhl, et al., Increased amount of zinc in the hippocampus and amygdala of Alzheimer’s diseased brains: a proton-induced X-ray emission spectroscopic analysis of cryostat sections from autopsy material, J. Neurosci. Methods 76, 53–59 (1997).
M. P. Cuajungco and G. J. Lees, Zinc and Alzheimer’s disease: is there a direct link? Brain Res. Rev. 23, 219–236 (1997).
G. Leblondel, Y. Mauras, and P. Allain, Tissue determination of some elements in rats, Biol. Trace Element Res. 10, 327–333 (1986).
S. J. McGregor, J. H. Brock, and D. Halls, The role of transferrin and citrate in cellular uptake of aluminum, Biol. Met. 4, 173–175 (1991).
J. B. Cannata, I. Fernandez-Soto, M. J. Fernandez-Menendez, J. L. Fernandez-Martin, S. J. McGregor, J. H. Brock, et al., Role of iron metabolism in absorption and cellular uptake of aluminum, Kidney Int. 39, 799–803 (1991).
G. F. Van Landeghem, P. C. D’Haese, L. V. Lamberts, and M. E. De Broe, Competition of iron and aluminum for transferrin: the molecular basis for aluminum deposition in iron-overloaded dialysis patients? Exp. Nephrol. 5, 239–245 (1997).
P. O. Ganrot, Metabolism and possible health effects of aluminum, Environ. Health Perspect. 65, 363–441 (1986).
W. Li, K. K. Ma, W. Sun, and H. K. Paudel, Phosphorylation sensitizes microtubule-associated protein tau to Al(3+)-induced aggregation, Neurochem. Res. 23, 1467–1476 (1998).
P. Evans and C. Harrington, Aluminosilicate particulate and beta-amyloid in vitro interactions: a model of Alzheimer plaque formation, Biochem. Soc. Trans. 26, S251 (1998).
L. Gandolfi, M. P. Stella, P. Zambenedett, and P. Zatta, Aluminum alters intracellular calcium homeostasis in vitro, Biochim. Biophys. Acta 1406, 315–320 (1998).
T. P. Flaten, A. C. Alfrey, J. D. Birchall, J. Savory, and R. A. Yokel, Status and future concerns of clinical and environmental aluminum toxicology, J. Toxicol. Environ. Health 48, 527–541 (1996).
J. C. Erickson, G. Hollopeter, S. A. Thomas, G. J. Froelick, and R. D. Palmiter, Disruption of the metallothionein-III gene in mice: analysis of brain zinc, behavior, and neuron vulnerability to metals, aging, and seizures, J. Neurosci. 17, 1271–1281 (1997).
D. W. Choi and J. Y. Koh, Zinc and brain injury, Annu. Rev. Neurosci. 21, 347–375 (1998).
J. W. Koh, S. W. Suh, B. J. Gwag, Y. Y. He, C. Y. Hsu, and D. W. Choi, The role of zinc in selective neuronal death after transient ischemia, Science 272, 1013–1016 (1996).
M. S. Golub, B. Han, C. L. Keen, M. E. Gershwin, and R. P. Tarara, Behavioral performance of Swiss Webster mice exposed to excess dietary aluminum during development or during development and as adults, Toxicol. Appl. Pharmacol. 133, 64–72 (1995).
G. Sahin, T. Taskin, K. Benli, and S. Duru, Impairment of motor coordination in mice after ingestion of aluminum chloride, Biol. Trace Element Res. 50, 79–85 (1995).
C. Struys-Ponsar, A. Kerkhofs, A. Gauthier, M. Soffie, and P. van den Bosch de Aguilar, Effects of aluminum exposure on behavioral parameters in the rat, Pharmacol. Biochem. Behav. 56, 643–648 (1997).
E. Alleva, J. Rankin, and D. Santucci, Neurobehavioral alteration in rodents following developmental exposure to aluminum, Toxicol. Ind. Health 14, 209–221 (1998).
M. W. King and M. C. K. Tsang, The fluoride content of Chinese and black teas available in Hong Kong, Fluoride 20, 18–23 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yang, M.S., Wong, M.H. Changes in Ca, Cu, Fe, Mg, and Zn contents in mouse brain tissues after prolonged oral ingestion of brick tea liquor containing a high level of Al. Biol Trace Elem Res 80, 67–76 (2001). https://doi.org/10.1385/BTER:80:1:67
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/BTER:80:1:67