Abstract
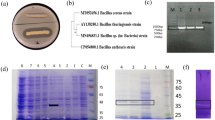
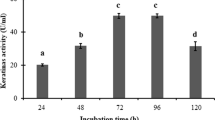
A keratinolytic protease-producing microorganism was isolated from soybean paste waste and was identified as a strain of Bacillus sp. The keratinase was purified by polyethylene glycol precipitation and two successive column chromatographies with DEAE-Toyopearl 650C and Sephacryl S-200 HR. The purified enzyme had overall 11 purification folds with an 18% yield. The results of sodium dodecyl sulfate polyacrylamide gel electrophoresis and gel filtration on Sephacryl G-200 indicated that the purified enzyme was monomeric and had a molecular weight of 134 kDa. The optimum temperature and pH were 40°C and 7.0, respectively. This enzyme was completely inhibited by EDTA and EGTA, and it was restored by the addition of Ca+2 and Mg+2. These results suggested that it is a metalloprotease. The stimulated enzyme activity by reducing agents indicated that the reducing condition was important in the expression of the activity.
Similar content being viewed by others
References
Moran, E. T. Jr., Summers, J. D., and Slinger, S. J. (1966), Poult. Sci. 45, 1257–1266.
Bahuguna, S. and Kushwaha, R. K. S. (1989), Mycoses 32, 340–343.
Rajak, R. C., Parwekar, S., Malviya, H., and Hasija, S. K. (1991), Mycopathologia 114, 83–87.
Kitadokoro, K., Tsuzuki, H., Nakamura, E., Sato, T., and Teraoka, H. (1994), Eur. J. Biochem. 220, 55–61.
Molyneaux, G. S. (1959), Aust. J. Biol. Sci. 12, 274–278.
Papadopoulos, M. C., El Boushy, A. R., Roodbeen, A. E., and Ketelaars, E. H. (1986), Anim. Feed Sci. Technol. 14, 279–290.
Papadopoulos, M. C., El Boushy, A. R., and Roodbeen, A. E. (1985), J. Sci. Food Agric. Abstr. 36, 1219–1226.
Dozie, I. N. S., Okeke, C. N., and Unaeze, N. C. (1994), World J. Microbiol. Biotechnol. 10, 563–567.
Onifade, A. A., Al-Sane, N. A., Al-Musallam, A. A., and Al-Zarban, S. (1998), Bioresour. Technol. 66, 1–11.
Williams, C. M., Richter, C. S., MacKenzie J. M. Jr., and Shih, J. C. H. (1990), Appl. Environ. Microbiol. 56, 1509–1515.
Williams, C. M. and Shih, J. C. H. (1989), J. Appl. Bacteriol. 67, 25–35.
Williams, C. M., Lee, C. G., Garlich, J. D., and Shih, J. C. H. (1991), Poult. Sci. 70, 85–94.
Shih, J. C. H. and Lee, C. G. (1993), US patent 5 186 961.
Hwang, J. H. (1996), PhD thesis, Korea University, Seoul, Korea.
Tomarelli, R. M., Charney, J., and Harding, M. L. (1949), J. Lab. Clin. Med. 34, 428–433.
Bradford, M. M. (1976), Anal. Biochem. 72, 248–254.
Laemmli, U. K. (1970), Nature 227, 680–685.
Moore, S. (1968), J. Biol. Chem. 243, 6281–6283.
Polson, A., Potgieter, G. M., Largier, J. F., Mears, G. E. F., and Joubert, F. J. (1964), Biochim. Biophys. Acta 82, 463–468.
Kula, M. R. (1990), Bioseparation 1, 181–189.
Lin, X., Lee, C., Casale, E. S., and Shih, J. C. H. (1992), Appl. Environ. Microbiol. 58, 3271–3275.
Bockle, B., Galunsky, B., and Muller, R. (1995), Appl. Environ. Microbiol. 61, 3705–3710.
Malviya, H. K., Tiwari, S., Rajak, R. C., and Hasija, S. K. (1993), Crypt. Bot. 3, 108–116.
Hahn, M. G., Darvill, A. G., and Albersheim, P. (1981), Plant Physiol. 68, 1161–1169.
Blanchard, J. S. (1984), Enzymology 104, 404–414.
Morihara, K., Tatsushi, O., and Tsuzuki, H. (1967), Biochim. Biophys. Acta 139, 382–397.
Bajorath, J., Hinrichs, W., and Saenger, W. (1988), Eur. J. Biochem. 176, 441–447.
Kunert, J. (1992), Mykosen 24, 485–496.
Takami, H., Nakamura, F., Aono, R., and Horikoshi, K. (1992), Biosci. Biotechnol. Biochem. 56, 1667–1669.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, H., Suh, D.B., Hwang, J.H. et al. Characterization of a kerationlytic metalloprotease from Bacillus sp. SCB-3. Appl Biochem Biotechnol 97, 123–133 (2002). https://doi.org/10.1385/ABAB:97:2:123
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/ABAB:97:2:123