Abstract
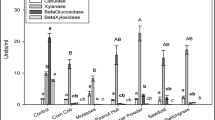
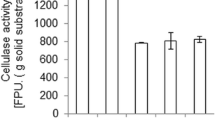
An aquatic weed biomass, Eicchornia crassipes, present in abundance and leading to a threatening level of water pollution was used as substrate for cellulase and β-glucosidase production using wild-type strain Aspergillus niger RK3 that was isolated from decomposing substrate. Alkali treatment of the biomass (10%) resulted in a 60–66% increase in endoglucanase, exoglucanase, and β-glucosidase production by the A. niger RK3 strain in semi-solid-state fermentation. Similarly, the alkali-treated biomass led to a 45–54% increase in endo- and exoglucanase and a higher (98%) increase in β-glucosidase production by Trichoderma reesei MTCC164 under similar conditions. However, the cocultivation of A. niger RK3 and T. reesei MTCC164 at a ratio of 3:1 showed a 20–24% increase in endo- and exoglucanase activities and about a 13% increase in the β-glucosidase activity over the maximum enzymatic activities observed under single culture conditions. Multistep physical (ultraviolet) and chemical (N-methyl-N′-nitrosoguanidine, sodium azide, colchicine) mutagenesis of the A. niger RK3 strain resulted in a highly cellulolytic mutant, UNSC-442, having an increase of 136, 138, and 96% in endoglucanase, exoglucanase, and β-glucosidase, activity, respectively. The cocultivation of mutant UNSC-442 along with T. reesei MTCC164 (at a ratio of 3:1) showed a further 10–11% increase in endo- and exoglucanase activities and a 29% increase in β-glucosidase activity in semi-solid-state fermentation.
Similar content being viewed by others
References
Kaya, F., Heitmann, J. A., and Joyce, T. W. (1996). J. Biotechnol. 45, 23–31.
Jackson, L. S., Joyce, T. W., Heitmann, J. A., and Giesbrecht, F. G. (1996), J. Biotechnol. 45, 33–44.
Sugden, C. and Bhat, M. K. (1994), World J. Microbiol. Biotechnol. 10, 444–451.
Teunissen, M. J., Lahaye, D. H. T. P., Veld, J. H. J. H., and Vogels, G. D. (1992), Arch. Microbiol. 158, 276–281.
Stahlberg, J., Johansson, G., and Pettersson, G. C. (1991), Biotechnology 9, 286–290.
Muniswaran, P. K. A. and Charyulu, N. C. L. N. (1994), Enzyme Microb. Technol. 16, 436–440.
Madamwar, D. and Patel, S. (1992), in Industrial Biotechnology, Malik, V. S. and Sridhar, P., eds., Oxford & IBH Publ., New Delhi, pp. 471–478.
Schwarz, W. H., Jauris, S., Kouba, M., Bronnenmeier, K., and Standenbauer, W. L. (1989), Biotechnol. Lett. 11, 461–466.
Singh, A., Abidi, A. B., Agrawal, A. K., and Darmwal, N. S. (1989), Folia Microbiol. 34, 479–484.
Nelson, N. and Oliver, D. W. (1971), J. Polym. Sci. Part C 36, 305–320.
Lakhani, A. (1990), PhD thesis, University of Roorkee, Roorkee, India.
Kuhad, R. C., Kumar, M., and Singh, A. (1994), Lett. Appl. Microbiol. 19, 397–400.
Reyes, L. M. and Noyola, T. P. (1998), Biotechnol. Lett. 20, 443–446.
Zohrer, E., Albertini, S., Gocke, E., and Knasmuller, S. (1996), Mut. Res. 356, 155–161.
Lotfi, C. F. P. and Santelli, G. M. N. (1996), Mut. Res. 349, 77–83.
Gupte, A. and Madamwar, D. (1997), Biotechnol. Prog. 13, 166–169.
Singh, A., Abidi, A. B., Darmwal, N. S., and Agrawal, A. K. (1991), Agric. Biol. Res. 7, 19–27.
Rajendran, A., Gunasekaran, P., and Laxmanan, N. (1994), Indian J. Microbiol. 34, 289–295.
Miller, G. L. (1959), Anal. Chem. 31, 426–428.
Menon, K., Rao, K. K., and Pushalkar, S. (1994), Indian J. Exp. Biol. 32, 706–709.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951), J. Biol. Chem. 93, 265–273.
Weil, J., Westgate, P., Kohlmann, K., and Ladisch, M. R. (1994), Enzyme Microb. Technol. 16, 1002–1004.
Koullas, D. P., Christakopoulos, P. F., Kekos, D., Koukios, E. G., and Macris, B. J. (1993), Biomass Energy 4, 9–13.
Arora, D. S. and Sandhu, D. K. (1986), Acta Biotechnol. 6, 293–297.
Pandey, A., Selvakumar, P., Soccol, C. R., and Nigam, P. (1999), Current. Sci. 77, 149–162.
Madamwar, D., Patel, S., and Parikh, H. (1989), J. Ferment. Technol. 67, 424–426.
Gutierrez-Correa, M. and Tengerdy, R. P. (1998), Biotechnol. Lett. 20, 45–47.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, R., Singh, R.P. Semi-solid-state fermentation of Eicchornia crassipes biomass as lignocellulosic biopolymer for cellulase and β-glucosidase production by cocultivation of Aspergillus niger RK3 and Trichoderma reesei MTCC164. Appl Biochem Biotechnol 96, 71–82 (2001). https://doi.org/10.1385/ABAB:96:1-3:071
Issue Date:
DOI: https://doi.org/10.1385/ABAB:96:1-3:071